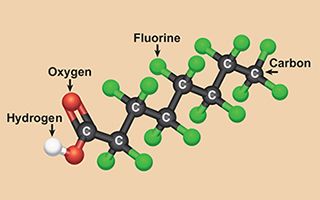
- PFAS are plastics that can resist water, heat, and grease, and are used in both industrial and commercial products; they’re often called “forever chemicals.”
- What are forever chemicals? They’re human-made molecules that build up in the environment without degrading, and even enter our bodies.
- Scientists are researching some promising ways to destroy these compounds, which can cause serious health problems.
When it encounters liquid-based fires, a foamy, white substance—called aqueous film-forming foam (AFFF)—is the most effective fire suppressant. It’s fantastic at snuffing out dangerous flames from jet fuel quickly; as such, the U.S. military and multiple organizations have been using the foam for decades.
Yet, every time a training exercise or an actual firefighting event employs AFFF, it adds to a problem that is much harder to extinguish. Once the substance is sprayed, it can’t be collected and disposed of safely—and on top of that, it’s toxic. Much of the material seeps into nearby groundwater, eventually making its way into the supply that permeates agricultural areas; freshwater rivers and lakes; and drinking water sources. Back in the 1970s, U.S. Air Force animal studies proved that this compound is toxic to the environment and to health. Yet, no good alternative exists for fighting liquid fires.
It gets worse. Fire-fighting foam is just one of literally thousands of materials made of human-made chemicals called Per- and Polyfluoroalkyl Substances (PFAS), often referred to as “forever chemicals.” Plastics that can resist water, heat, and grease, PFAS were invented in the 1950s, and since then have been used for a wide range of applications. They show up as a non-stick coating for cooking pans, textiles, and paper products. They fulfill the needs of industries ranging from aerospace to semiconductors to automotive.
Eventually, PFAS microparticles accumulate in water, making its way into crops, fish, and other meat. These particles are so pervasive that they’re also present in the dust on your bookshelf at home, and you can even inhale it. Studies have found that just about everyone on the planet today may have detectable levels of two insidious PFAS types in their blood: perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA).
It seems there is no getting away from them. So what are these forever chemicals, anyway?
What Are Forever Chemicals?
A long-chain compound, most PFAS chemicals are now known colloquially as forever chemicals. That’s because they refuse to break down. Chronic exposure, especially from swallowing PFAS, is linked to many health problems, including decreased fertility; hormonal changes; increased cholesterol; weakened immune system response; increased risk of some cancers; and growth and learning delays in infants and children, according to the U.S. Centers for Disease Control and Prevention.
The struggle to eradicate PFAS from the environment has ramped up in the past few years. Researchers have only begun to study some alternatives to the harsh methods used so far, like incineration. Actually, the immense heat (about 400 degrees Celsius) and pressure required to break these compounds leads to air contamination, spraying PFAS out of airstacks. You could almost compare PFAS to the indestructible One Ring from Tolkien’s The Lord of the Rings epic; one character tells another gravely, “... As for breaking the Ring, force is useless. Even if you took it and struck it with a heavy sledge-hammer, it would make no dint in it … there is no smith’s forge … Not even the anvils and furnaces. ...There is only one way: to find the Cracks of Doom.”
Over the past decade or so, a growing number of scientists have been studying PFAS molecules, seeking an alternative approach for breaking them down. So far, fire won’t reduce it to ashes; other chemical agents can’t break PFAS; and even bacteria, some of which are known to consume certain artificial compounds, fail to do the job.
One chemistry researcher, whose goal is to find a practical way to destroy PFAS, is William Dichtel, a professor at Northwestern University. “There are literally tens of thousands of products that contain PFAS. And as far as I’m concerned, there are very, very few—if any—consumer-facing products that really should have them, based on what we know about their toxicity and their persistence at this point,” Dichtel tells Popular Mechanics. His team has developed one of the most promising techniques so far, and published a paper on its findings in the journal Science in August 2022. It turns out that the “Cracks of Doom”—for about ten PFAS compounds, at least—is a combination of low temperatures and inexpensive, common reagents.
Breaking the Strong Bonds of PFAS
PFAS molecules are made of a long chain of carbon and fluorine atoms linked together in an incredibly strong bond. They often include charged oxygen atoms at one end of the chain. Dichtel’s team discovered that these atoms are the PFAS molecule’s weak spot.
Researchers added an uncommon solvent, dimethyl sulfoxide, to heat the molecule to between 80 and 120 degrees Celsius. Sodium hydroxide, a typical reagent that helps cause a chemical reaction, was also part of the solution. This magic combination forced the charged oxygen atoms to fall away, which then prompted the fluorine atoms to separate as well. They left their carbon companions alone, forming the safe fluorine form called fluoride—which is in the stuff dentists put on kids’ teeth to strengthen enamel. The whole process took only 12 hours, by which time, more than 90 percent of the PFAS chemicals in the study were converted into safe carbon byproducts. Dichtel called the group of charged atoms the PFAS molecule’s “Achilles’ heel” in a 2022 Northwestern news release. Water treatment plants in high-risk places would be the predominant targets for solutions like Dichtel’s.
More From Pop Mech:
- 🤖 This Recycling Tech Is Battling the Climate Crisis
- 💧 Study Finds Almost All Drinking Water Is Contaminated With Plastic
- 🌊 Tiny, Plastic ‘Nurdles’ Are Contaminating Our Shores. Here’s How You Can Help
Mechanical destruction has potential, too. Dichtel’s group also published a paper on a technique to break down PFAS using high mechanical force to break these things down, he says. “Some of these ways will turn out to be scalable, so that bodies of freshwater and drinking water can actually be cleaned,” he explains.
Other techniques, like supercritical water oxidation, appear promising as well, Dichtel says. This technology—which nonprofit company Battelle uses to clean water contaminated by AFFF and other kinds of PFAS, for example—starts with water above its “critical point,” heated to 374 degrees Celsius and pressurized to 22 megapascals. The process breaks PFAS molecules into smaller pieces that include hydrofluoric acid. Sodium hydroxide neutralizes the acid to form non-toxic salts and PFAS-free water.
Regulation Lags Far Behind the Facts
About 12,000 different PFAS compounds exist today, and most of them are bad for people and the environment, while also accumulating without degrading. (Only two types of PFAS, polyvinyl fluoride [PVF] and polytetrafluorethylene [PTFE], break down eventually.)
Companies such as 3M and DuPont (the latter of which conducts its PFAS business under the name Chemours) have started removing known toxic PFAS compounds from their products at the government’s directive. Yet in many cases, says Dichtel, these same companies simply replace the forbidden compound with another PFAS compound. In the story of plastic, PFAS is the villain that keeps coming back, refusing to die.
PFAS as a whole must be regulated as a single class of chemicals, not piecemeal, as it is now, Dichtel says. Each individual compound is studied and determined to be toxic, a long process that disregards the continued use of thousands of other PFAS compounds.
“Removing it from water is just one piece of the problem. And it’s an important piece of the problem. But, you know it’s not going to be the silver bullet, right? The final solution is going to be dramatically curtailing PFAS use, limiting it only to the absolutely essential societal functions, things like semiconductor manufacturing,” Dichtel says. He is among a growing number of scientists who say PFAS use should be restricted. “We need to remove it from consumer-facing products, we need to destroy it where we can, but mostly, we need to stop using it. Then, this problem will slowly get better.”
Are You at Risk of Consuming PFAS Where You Live?
On June 15, 2022, the Environmental Protection Agency (EPA) issued interim updated drinking water health advisories for PFOA and PFOS. Communities based close to areas where the compound is heavily used tend to be adversely affected by PFAS contamination.
At the moment, “heavily contaminated communities are often using activated carbon as an absorbent to bring down the concentrations of PFAS compounds, as we’re realizing just how toxic they are. And that’s a very expensive treatment,” says Dichtel.
You can find out if your drinking water has PFAS using this EPA resource.
Eating freshwater fish is also a risk. An Environmental Working Group (EWG) study found that eating one freshwater fish equals a month of drinking “forever chemicals” water.
If you enjoy fishing for your own dinner, you should check this map to make sure your catch is PFAS-free. The Environmental Protection Agency tested more than 500 freshwater fish samples across the U.S. from 2013–2015.
Before joining Popular Mechanics, Manasee Wagh worked as a newspaper reporter, a science journalist, a tech writer, and a computer engineer. She’s always looking for ways to combine the three greatest joys in her life: science, travel, and food.