This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers describe promising method to optimize and scale up the production of fuel cell input
A novel process described in an article published in the journal Electrochemistry Communications converts nitrogen and hydrogen to ammonia (NH3) at ambient temperature and pressure with high energy efficiency. Ammonia, a gas composed of nitrogen and hydrogen, is the world's most synthesized molecule and is used in agriculture as well as many production processes.
It does not emit CO2 when burned and is expected to become a next-generation fuel as it contains properties ideally suited for the hydrogen economy. Annual output of ammonia amounts to about 1.2 million metric tons.
In response to growing interest in decarbonization routes, researchers have recently turned their attention to the use of ammonia in fuel cells—electrochemical or galvanic cells that generate electricity from specific chemical reactions, such as the nitrogen reduction reaction (NRR).
NRR electrochemistry is studied with two main goals: production of a key industrial input and potential future fuel; and storing hydrogen in a molecule—ammonia—that can easily be diluted in water and transported more safely and cheaply than hydrogen alone. If ammonia fuel cell research initiatives are successful, global demand for ammonia will grow even more strongly.
The group used an electrochemical reactor to outperform the Haber-Bosch process, which releases large amounts of heat into the environment. Haber-Bosch is the primary industrial method of producing ammonia from nitrogen and hydrogen.
"In our reactor, the process occurs through electron-nucleus interactions and dissipates much less energy," said Rodrigo Fernando Brambilla de Souza, last author of the article. He works with a scholarship at the Advanced Energy Storage Division of the Center for Innovation in New Energies (CINE).
Launched in May 2018, CINE conducts research in partnership with three academic institutions in São Paulo state: the University of São Paulo (USP), the State University of Campinas (UNICAMP), and the Nuclear and Energy Research Institute (IPEN).
Several research groups have been looking for ways to produce ammonia under ambient conditions. Conventional electrochemical processes use liquid electrolytes, which are efficient for synthesis purposes but contaminate the ammonia, so that a purification step is required.
"Our process uses a solid polymeric electrolyte, and the anode oxidizes hydrogen to provide protons, which bond chemically with nitrogen molecules adsorbed by the copper catalyst. The output is ammonia gas, eliminating the need for purification to remove the electrolyte," Souza explained. Adsorption involves adhesion of molecules of a gas, liquid or dissolved solid (the adsorbate) to a surface (the adsorbent).
"Another advantage is that the reactor operates in continuous flow. Nitrogen and hydrogen are delivered to the electrodes, and the ammonia is produced and released continuously," said Souza, who has a Ph.D. in chemistry from the Federal University of the ABC (UFABC) and is conducting postdoctoral research at IPEN.
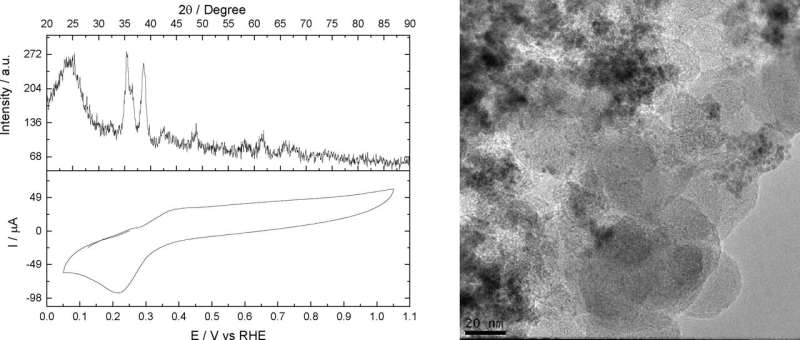
"The article shows real-world results of operating a different type of reactor in fundamental electrochemical studies that don't always represent real results. It's important to note that the ammonia obtained in our study is pure, whereas in other studies there have been impurities," said Almir Oliveira Neto, penultimate author of the article. He has a Ph.D. in physical chemistry from USP and is also a postdoctoral researcher at IPEN.
More information: Victoria A. Maia et al, Conversion of nitrogen to ammonia using a Cu/C electrocatalyst in a polymeric electrolyte reactor, Electrochemistry Communications (2022). DOI: 10.1016/j.elecom.2022.107421
Journal information: Electrochemistry Communications
Provided by FAPESP