The inherited retinal disease, retinitis pigmentosa (RP)—marked by progressive and irreversible loss of retinal photoreceptors—is a major cause of blindness in humans.
Now, researchers have successfully restored the vision of mice with retinitis pigmentosa using a genome editing technique they developed (referred to as PESpRY) characterized by the versatility of prime editors and unconstrained PAM requirement of a SpCas9 variant (SpRY.)
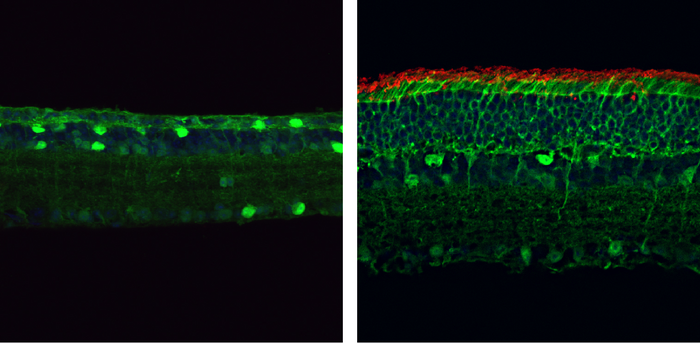
The diseased retinas of the RP mouse model were transduced using an AAV system packaging PESpRY for the in vivo genome editing. The progressing cell loss was reversed once the mutation was corrected, leading to substantial rescue of photoreceptors. Moreover, the mice performed well in vision tests such as visually guided water-maze tasks.
This research is published in the Journal of Experimental Medicine in the article, “Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas.”
“The ability to edit the genome of neural retinal cells, particularly unhealthy or dying photoreceptors, would provide much more convincing evidence for the potential applications of these genome-editing tools in treating diseases such as retinitis pigmentosa,” said Kai Yao, PhD, professor at the Wuhan University of Science and Technology.
Researchers have previously used genome editing to restore the vision of mice with genetic diseases (such as Leber congenital amaurosis) that affect the retinal pigment epithelium—a layer of non-neuronal cells in the eye that supports the light-sensing rod and cone photoreceptor cells. However, most inherited forms of blindness, including retinitis pigmentosa, are caused by genetic defects in the neural photoreceptors themselves.
Retinitis pigmentosa can be caused by mutations in over 100 different genes and is estimated to impair the vision of 1 in 4,000 people.
Yao and colleagues attempted to rescue the vision of mice with retinitis pigmentosa caused by a mutation in the gene encoding PDE6β. To do this, Yao’s team developed the CRISPR system called PESpRY, which can be programmed to correct many different types of genetic mutation, regardless of where they occur within the genome.
When programmed to target the mutant PDE6β gene, the PESpRY system was able to efficiently correct the mutation and restore the enzyme’s activity in the retinas of mice. This prevented the death of rod and cone photoreceptors and restored their normal electrical responses to light.
Yao and colleagues performed a variety of behavioral tests to confirm that the gene-edited mice retained their vision. For example, the animals were able to find their way out of a visually guided water maze almost as well as normal, healthy mice and showed typical head movements in response to visual stimuli.
Much work still needs to be done to establish both the safety and efficacy of the PESpRY system in humans. “However, our study provides substantial evidence for the in vivo applicability of this new genome-editing strategy and its potential in diverse research and therapeutic contexts, in particular for inherited retinal diseases such as retinitis pigmentosa,” Yao said.