Pathology
Biopsy Findings
Although the symptoms of CADASIL are almost exclusively neurological, blood vessels (mainly medium sized and small arteries) throughout the body are affected (Figures 4-6). A pathognomonic feature of CADASIL is the presence of GOM (Figures 4, 5A & 5B) detectable with electron microscope (EM) on the VSMCs in the walls of the affected vessels. GOM was originally demonstrated by Baudrimont et al. and Ruchoux et al.[63,64] and it has not been detected in any other disease. GOM is located either in indentations on the degenerating VSMCs or free between these cells, the basal lamina of which is usually irregularly thickened (Figures 4, 5A & 5B). Both accumulation of GOM and degeneration of VSMCs are detectable already before 20 years of age (Figure 5B).[65,66] The exact composition of GOM is not yet clear, but on the basis of immunoelectron microscopy N3ECD has been suggested to be a major component of GOM.[67] By light microscopic immunohistochemistry with antibodies against N3ECD, introduced by Joutel et al. , the vessel walls stain diffusely positively (Figure 6A).[68] With confocal microscopy N3ECD immunoreactivity appears as dot-like accumulations on the surface of VSMCs, in concordance with the appearance of GOM deposits in EM (Figure 6B).
Figure 4.
Electron micrograph of a dermal artery from a patient at an early stage of the disease. A 28-year old male with p.Arg133Cys mutation. Note the widened subendothelial spaces (asterisks) and irregular smooth muscle cells (M) on which there are three deposits of granular osmiophilic material (arrows).
E: Endothelial cell.
Figure 5.
Electron micrograph showing presence of granular osmiophilic material.
(A) In a 48-year-old patient who had already suffered from a couple of strokes relatively abundant granular osmiophilic material (GOM; asterisks) is present in the indentations of two vascular smooth muscle cells. (B) A small amount of GOM is already present in a 19-year-old patient carrying the p.Arg133Cys mutation. GOM is located in a deep invagination, which might be difficult to detect by immunohistochemistry.
Figure 6.
Since CADASIL is a generalized arteriopathy, its characteristic pathological alterations are also visible in skin biopsy.
(A) A 57-year-old female with p.Arg133Cys mutation. N3ECD is present in the wall of the small dermal arteries (asterisks). (B) A 62-year-old male with p.Tyr1069Cys mutation. Confocal microscopy demonstrates N3ECD immunoreactivity as small dots along the vessel wall, a finding compatible with the deposits of GOM (see Figures 4 & 5).
Figure 6B is courtesy of Dr Ismo Virtanen.
Autopsy Findings
Macroscopy. In accordance with the imaging findings, there are multiple small (lacunar) infarcts in the WM and/or deep GM, whereas the cerebral cortex is markedly well preserved (Figure 1). Lacunar infarcts are also relatively common in the brain stem. Even though the microbleeds are fairly common (see above section, Imaging), parenchymal brain hemorrhages are relatively uncommon. These have most often occurred in patients treated with anticoagulants or antiaggregants, or subjected to arteriography.
Histopathology. Histological stainings reveal markedly thickened small arteries in WM with accumulation of granular material in the degenerating tunica media. This material is basophilic in hematoxylin and eosin and red in periodic acid-Schiff stainings (Figures 7A-7C). Decreased immunopositivity for α-smooth muscle actin reveals degeneration of the VSMCs (Figure 7D). The accumulation of N3ECD may be verified by immunohistochemical staining (Figure 7E). It is the accumulation of extracellular matrix proteins, including various types of collagens and laminin outside the degenerating VSMCs, that causes the thickening of the vessel walls (Figure 7F). On the basis of the stainings described above, CADASIL can be distinguished from the two other arteriopathies with thickened walls: in arteriolosclerosis (Binswanger disease) and cerebral amyloid angiopathy, the walls are homogeneously stained, either collagen or similar to amyloid.
Figure 7.
Histopathological findings in CADASIL patients' brain.
(A) A small arteriole from the cerebral white matter of a control person. (B) The wall of a corresponding arteriole from a CADASIL patient is markedly thickened and the lumen stenosed. Note the basophilic granular material in the thick media and adventitia. (C) The granular material in B is PAS-positive. (D) The degeneration of vascular smooth muscle cells (VSMCs) appears either as almost complete loss of α-SMA-stainable VSMCs (left arteriole) or their irregular pattern (right arteriole). (E) N3ECD accumulates in the tunica media of affected arterioles. (F) The degenerated VSMCs have been replaced by abundant collagen I.
α-SMA: α-smooth muscle actin; N3ECD: Notch3 extracellular domain; PAS: Periodic acid-Schiff.
The marked thickening of the walls of small penetrating arteries in cerebral WM is reflected numerically in the highly significant increase of sclerotic index (SI = 1-internal diameter/external diameter; 0.75 vs 0.35-0.40 in controls).[69] Within WM, the small arterioles (<130 µm external diameter) are most severely affected, and at the same time they become stenosed with significantly smaller mean internal (luminal) diameters (Figures 8 & 9).[69] Finally, these arterioles must become severely enough obliterated or thrombosed to give rise to lacunar infarcts in cerebral WM (Figures 1 & 8). In the cerebral cortex, the small arteries and arterioles are also thickened (SI: 0.56 vs 0.48 in controls) and their lumina somewhat narrowed (Figure 9), but to a considerably lesser extent than in WM, explaining the absence of cortical infarcts.[69] Corresponding to the presence of the microbleeds in imaging, perivascular deposits of hemosiderin may be found.[54] These are located predominantly in GM, which may simply depend on the fact that the walls of arterioles in GM are less thickened than in WM and therefore more fragile[69] and prone to extravasation.
Figure 8.
Progressive fibrosis of the affected arterioles in cerebral white matter may lead to complete obliteration of the affected arteriole. Note the loose texture of the surrounding infarcted tissue.
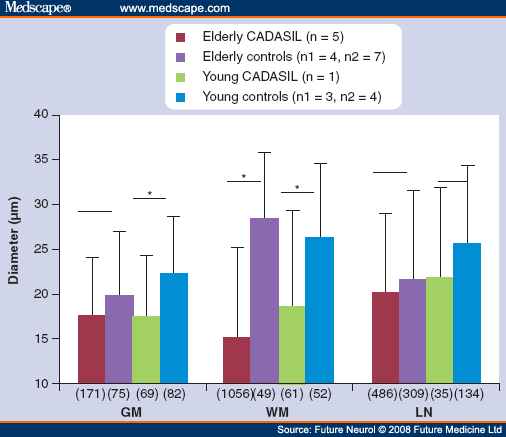
Figure 9.
Mean diameters of arteriolar (0-50 µm) lumina demonstrate the stenosis occurring in CADASIL patients, most marked in the cerebral white matter and at more advanced stage. Horizontal lines above the columns indicate the two groups compared. The asterisks above the lines indicate statistical significance. The numbers of arterioles measured are given in brackets under each column.
CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy; GM: Grey matter; LN: Lentiform nucleus; n: Numbers of CADASIL patients (elderly with pArg133Cys mutation, mean age: 63.4 ± 2.9 years, range 60-68 years, young with pCys174Arg mutation, age 32 years); n1: Numbers of controls for GM and WM ; n2: Numbers of LN controls; WM: White matter.
Interestingly, the small arteries in the deep gray matter of basal ganglia (nucleus lentiformis ) - the other region in the brain, where lacunar infarcts occur in CADASIL - behave differently. These vessels are not stenosed (Figure 9) as in WM even though their walls are thickened, suggesting a different, most probably hemodynamic, pathogenesis for the lacunar infarcts in the basal ganglia.[70]
These findings suggest that there is a fundamental difference between the cortical and WM arteries. The response of VSMCs to the gene defect appear to be markedly different, which is also reflected in the magnitude of the fibrotic reaction. Similarly, it was recently shown in NOTCH3 -knockout mice that the contractile activity of VSMCs in the aorta is different from that of cerebral arteries.[71]
Since NOTCH3 is mainly or exclusively expressed in VSMCs, the main interest has been targeted at these cells and their degeneration. Ruchoux and Maurage have performed the most detailed structural analysis of endothelium in skin and skeletal muscle biopsies.[72] They found attenuation of endothelial cells and increased density of their cytoplasm with accumulation of microfilament bundles. Widening of subendothelial space has also been observed (Figure 4). Interaction between endothelial and vascular smooth muscle cells has been identified as a central process in the regulation of vascular formation, stabilization, remodeling and function.[73] During development, expression of Notch3 signaling appears to be necessary for correct specification of arterial and venous characteristics of the VSMCs in the blood vessel walls.[74] Thus, the pathological findings in CADASIL patients' endothelium may reflect functional disturbances: we have shown impaired endothelium-dependent vasodilation in CADASIL patients' forearm resistance arteries,[61] and in addition, other studies have suggested endothelial dysfunction.[75]
Future Neurology. 2008;3(6):683-704. © 2008 Future Medicine Ltd.
Cite this: CADASIL: The Most Common Hereditary Subcortical Vascular Dementia - Medscape - Nov 01, 2008.














Comments