Point-of-care blood glucose monitoring systems are known to provide an accurate, timely, and cost-effective means for determining blood glucose levels in diabetic patients.[1] In fact, these devices have become a mainstay for blood glucose monitoring in both hospitals and outpatient settings. In many cases, this has allowed patients to achieve better glycemic control and avert the negative health outcomes associated with hypoglycemia or hyperglycemia. However, patients and practitioners may not know that the administration of certain substances can result in erroneously high values when using glucose meters. One situation was recently reported.
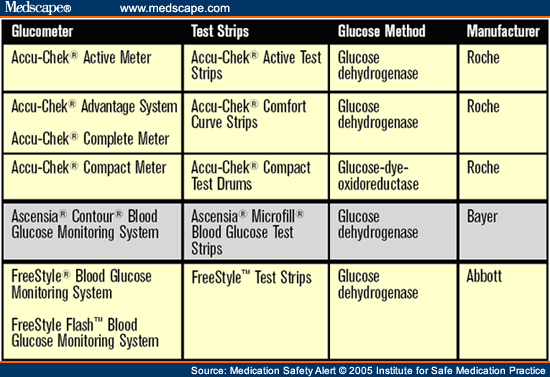
An elderly immune-compromised patient was admitted to a hospital ICU with sepsis. He was started on intravenous immunoglobulin treatment with OCTAGAM (immune globulin intravenous [human] 5% solvent/detergent treated). The hospital routinely stocked another manufacturer's immunoglobulin preparation but recently switched to Octagam. The manufacturer of Octagam has added maltose (100 mg/mL) to make it isotonic with plasma, but this substance can also interfere with certain blood and urine glucose tests. The ICU staff were unaware that maltose could cause falsely elevated glucose values in glucose meters that use the glucose dehydrogenase method for testing (see table 1 ). The package inserts for glucose dehydrogenase test strips and Octagam describe this problem, but the information was overlooked. To test the patient's blood, nurses were using an Accu-Chek system and Accu-Chek Comfort Curve test strips, which use the glucose dehydrogenase method for determining glucose levels. Thus, the Accu-Chek provided falsely elevated glucose values, which were used to adjust the patient's insulin doses according to an aggressive insulin protocol. The patient was soon receiving an insulin infusion at 24 units/hour. Because the patient was actually receiving too much insulin, he experienced profound hypoglycemia, which was confirmed with a laboratory-drawn glucose level of 12 mg/dL. The patient also developed irreversible neurological damage, although this outcome could not be definitively linked to the patient's severe hypoglycemia.
Regulatory agencies in Australia, Canada, and the United Kingdom have received similar reports describing this substance-device interaction.[2,3,4] These agencies found that maltose interacts with numerous test strips, including those used with certain Accu-Chek (Active, Comfort Curve, and Compact) and Ascensia (Microfill) systems, as well as Freestyle test strips (see table 1 ). This interaction has resulted in false readings of hyperglycemia and unnecessary insulin administration, sometimes leading to injury and death. Additionally, falsely elevated glucose readings could mask true hypoglycemia, which also could result in fatal consequences if left untreated.
Other products could lead to false glucose readings if maltose is involved. One example is EXTRANEAL (icodextrin 7.5%), a peritoneal dialysis solution. Icodextrin is metabolized to maltose and other oligosaccharides following peritoneal absorption. Thus, if the glucose test strips use a glucose dehydrogenase method for testing, patients who receive Extraneal may exhibit falsely high glucose readings. A general lack of awareness among practitioners of potential device interactions with maltose, along with incomplete knowledge of product formulations, also contributes to the risks involved with erroneous glucose meter readings.
Consider the following suggestions to reduce the risk of reliance upon potentially false glucose meter readings from point-of-care systems.
Create special alerts in the pharmacy computer system to remind pharmacists about the potential for false glucose readings when entering orders for products that may contribute to the problem. Communicate the information to the nursing staff and add a cautionary note to the medication administration record under the drug entry for insulin.
Increase staff awareness about the potential for false glucose values so they stay alert to the possibility and detect such a situation as quickly as possible. Include all staff who might perform blood glucose testing at the point-of-care. If involved products are used infrequently, a table posted in areas where insulin is prepared might be helpful.
Include the risk of false glucose determinations, and the specific circumstances surrounding this risk, in protocols for insulin administration according to glucose values. Under these circumstances, consider the need for laboratory testing of glucose levels, or the use of glucose meters that employ the glucose oxidase method of determining glucose levels. Also include symptoms and other monitoring criteria besides glucose values that can be used to verify hypoglycemia and hyperglycemia.
Remind staff to "treat the patient, not the glucose meter." Ask them to always correlate the patient's symptoms with the glucose meter reading before insulin administration, especially in cases in which the glucose readings are very high or very low.
Establish written guidelines for early recognition of an error (e.g., discrepancies between laboratory and glucose meter readings taken in close time proximity, symptoms of hypoglycemia or hyperglycemia), and prompt recourse in the event an error has occurred. These include required periodic reconciliation of laboratory and glucose meter readings whenever an insulin drip is infusing, steps to verify a suspicious glucose meter reading, and procedure updates for an immediate response whenever patients exhibit signs of hypoglycemia or hyperglycemia.
If there is a substance-device interaction, let patients know why the glucose meter or certain test strips cannot be used for testing their blood glucose levels. Tell patients to alert any staff member who may attempt to use a glucose meter during the course of their treatment using the involved substance.
Medications may have the same active ingredients, concentrations, and volume, but still differ significantly in formulation from those produced by other manufacturers. Since differences in preservatives, salts, excipients, and other inactive substances may have serious implications, evaluate all new products and changes in product formulation for these aspects before purchase and use.
Labeling changes for glucose meters, test strips, and substances that may interfere with proper performance should be undertaken to more clearly identify potential interactions. Language that clearly describes the magnitude of falsely high glucose levels, and injuries that have occurred from interactions, should be included.
© 2005 Institute for Safe Medication Practices
Cite this: Be Aware of False Glucose Results With Point-of-Care Testing - Medscape - Sep 01, 2005.