Implant-related Allergic Contact Dermatitis
The most common types of cutaneous allergic reactions associated with metallic implants are eczematous in nature, although urticaria and vasculitis have occasionally been reported.[7,9] Eruptions may be localized or generalized or both. Localized eruptions present as dermatitis primarily affecting the skin overlying the site of the implant. Generalized eruptions most often present as eczematous reactions and occur equally in association with static and dynamic implants. Various diagnostic criteria have been proposed for implant-induced cutaneous allergic reactions. The most recent criteria were proposed in 1992 and are included in Table 3.[19] We are currently developing an updated approach.
The few prospective longitudinal studies that have examined the association between metal sensitivity and cutaneous allergic reactions are summarized in Table 4. The first study was performed by Carlsson and Möller in 1989.[20] A series of 18 patients were identified as metal allergic prior to receiving stainless steel orthopedic implants. None of the 18 patients who were observed for up to 6 years had complications despite confirmed allergy to one of the metals in his or her device. Later studies suggest that up to 5% of all patients with orthopedic implants and up to 21% of patients with preoperative metal sensitivity may develop cutaneous allergic reactions upon reexposure to the same metal.[21] More longitudinal prospective studies are needed to better define the actual prevalence of implant-induced reactions and determine whether metal-allergic subjects have an increased risk of complications. In Germany, national databases are currently being created to better study the association between metal allergy and implant failure.[22]
Allergic Contact Dermatitis and Extracutaneous Complications from Orthopedic Implants
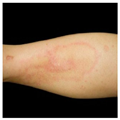
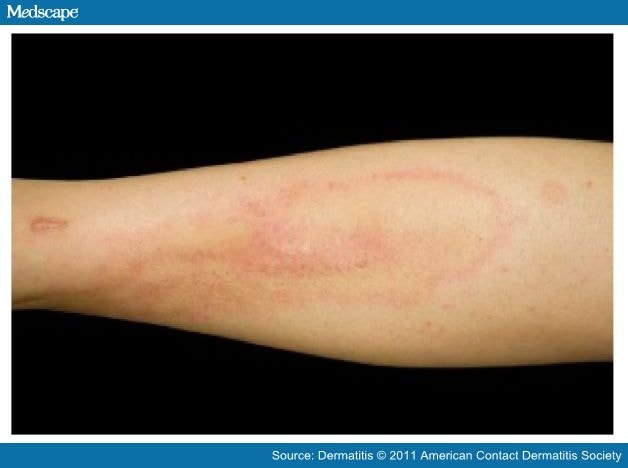
The first case of metal-related dermatitis was reported in 1966;[23] since then, a growing number of reports of such cases have been published in the literature.[8,17,21,24–28] By 1986, 42 such cases had been documented; 30 patients developed dermatitis in the setting of a static implant, whereas the remaining 12 patients with dermatitis had received a dynamic joint prosthesis.[24] The condition of 18 (42.9%) of the 42 patients was diagnosed as "eczematous dermatitis." Generalized eruptions in the form of erythema, [29] urticaria,[30] and vasculitis were also reported.[19,31] An example of dermatitis adjacent to a static titanium implant is shown in Figure 1.
Figure 1.
The shin of a woman with dermatitis adjacent to implanted titanium orthopedic hardware. Pathology examination revealed perivascular and periadnexal lymphoeosinophilic infiltrates consistent with hypersensitivity reaction. This resolved within 3 weeks of the hardware's removal.
A non-exhaustive summary of reported cases of cutaneous reactions caused by a metallic implant is given in Table 5. Although many of the patients were patch test positive to their implanted metals, it is important to note that several were patch test negative. Although neither lymphocyte transformation test results nor serum metal levels were reported in these cases, those examinations may be useful in confirming metal allergy in these types of patients. The temporal and physical evidence before and after removal of the implants leaves little doubt that a considerable number of patients develop metal sensitivity and cutaneous allergic dermatitis in association with metallic orthopedic implants.
Noncutaneous complications involving orthopedic implants may occur following total hip or knee arthroplasty as well as following the insertion of other dynamic implants. Cases of noncutaneous reactions believed to be caused by metal hip and knee implants are summarized in Table 6 and Table 7. First-generation metal-on-metal orthopedic hip bearings were introduced in the 1960s and 1970s and were associated with high rates of metal release and sensitization (28–46%).[11,32] The prostheses resulted in the excessive release of cobalt, nickel, and chromium into the blood, hair, and urine.[33,34] Metal-on-plastic implants, which were increasingly used from the 1970s through the 1990s, are less likely to induce metal sensitization because they release large polyethylene wear particles that prevent the formation of allergenic polymer-protein complexes.[13,35]
Recently, second-generation metal-on-metal bearings were introduced. Such prostheses have a lower volumetric wear rate, high fracture toughness, and the ability to use large femoral heads, which may decrease the risk of postoperative instability.[36] These bearings are typically used with younger patients. However, a few studies have documented elevated serum and urine concentrations of cobalt and chromium as seen with first-generation metal-on-metal hip bearings.[36–40] A recent case-control study comparing the prevalence of complications following hip arthroplasty in patients with and without a previous metal allergy found no overall difference.[41] Also, clinically serious complications with aseptic lymphocytic vasculitis– associated lesions and pseudotumors have been reported, typically in association with metal-on-metal bearings (see Table 6 and Table 7).
To what degree metal sensitivity contributes to implant failure remains highly controversial. Thomas and colleagues[42] studied a cohort of 16 patients with failed metal-on-metal arthroplastic implants; 81% of the patients were found to have metal sensitivity (defined as a positive patch-test reaction or positive lymphocyte transformation test result or both), suggesting that metal hypersensitivity may be contributing to the failure of metal-on-metal arthroplastic implants. Reed and colleagues[43] studied 44 patients, 22 of whom had a history of metal reactions evaluated prior to metal implantation and 22 of whom had the following symptoms following implantation: unexplained skin eruptions at the implantation site (13 patients), chronic joint pain (8 patients), and joint loosening (1 patient). None of the symptomatic patients had had positive patch-test reactions to a component of the implanted device. In the preimplantation group, 5 of 22 patients had metal sensitivity, resulting in avoidance of the material in the implant. The authors suggested that preimplantation patch testing might be useful for evaluating the cases of those patients who have a reported history of metal sensitivities. Savarino and colleagues[44] examined 59 patients who had total knee replacements (24 stable and 35 loosened) and compared their measured serum levels of aluminum, titanium, chromium, and cobalt ions to those of 41 healthy controls. Chromium ion levels were significantly elevated (p = .001) in those with loosened implants. The other metal ions were not significant.
Accumulated reports of metal allergy in total hip arthroplasty patients showed that the prevalence of metal allergy was approximately 25% among patients with a well-functioning hip arthroplastic implant and 60% among patients with a failed or poorly functioning implant.[9] Despite these prevalences' being much higher than general population estimates, it is uncertain whether metal allergy causes device failure or whether device failure causes metal allergy.
Allergic Contact Dermatitis from Bone Cement Components
Allergic contact dermatitis from bone cement components may also occur and was reported in 24.8% of patients in one series (n = 239).[45] Most orthopedic bone cements are composed of methyl methacrylate (MMA), N,N-dimethylp-toluidine (DPT), and benzoyl peroxide.[46] Antibiotics (gentamicin being the most prevalent) are often added to the cement. These cements may contain tobramycin, clindamycin, and erythromycin.[46] DPT may be a significant cause of aseptic loosening. In one series, 7 of 15 patients with aseptic loosening of a total hip replacement were DPT allergic.[47]
Although MMA is a known allergen for orthopedic and dental workers, the relevance of MMA as a cause of allergy in joint replacement is not well defined in the literature. In one study, a single series of 42 patients was patch-tested with MMA 6 months after hip arthroplasty; 25% of this cohort had positive reactions to MMA.[48]
The most common components that cause potential orthopedic joint symptoms and potential failure are listed in Table 8. The addition of these agents to any screening patch test is recommended.
Allergic Contact Dermatitis from Dental Implants and Prostheses
Cases of allergic contact dermatitis in association with dental implants have also been reported.[9,11,13,49–56] In 1966, Foussereau and Langier[23] reported a case of generalized dermatitis in the setting of a chromium-nickel denture. Patch testing elicited a strong reaction to nickel and chromium in this patient. The skin eruption resolved completely after the denture was removed. Hubler and Hubler[11] reported a similar case of generalized eczema following the placement of a denture plate that contained a chromium-cobalt alloy. Removal of the dental plate cleared the eruption, but the eruption reappeared within 24 hours of the denture plate's reinsertion. Pigatto and colleagues[56] described a 48-year-old atopic woman who developed generalized eczematous dermatitis after the placement of titanium dental implants and (later) a dental prosthesis containing chromium-cobalt alloy. Patch testing revealed allergies to dental amalgam, nickel sulfate, and palladium chloride.
Allergic contact dermatitis from dental implants may present differently in individual patients. The most frequent manifestation is a lichenoid reaction characterized by oral lichen planus–like lesions. The lesions may be reticular, atrophic, erosive, or plaquelike and usually abut the eliciting implant. Lichenoid reactions have been reported in association with dental amalgams and gold.[57]
Mercury amalgams, the most commonly used restorative material in dental practice, release large quantities of mercury ions. Mercury ions are the most frequent potential allergens that induce a cell-mediated DTH reaction. Other metals (including copper, zinc, palladium, cobalt, and tin) have also been implicated in eliciting contact allergy. Lichenoid reactions due to gold sensitization have been reported, albeit less frequently.[58–64] The use of amalgam fillings has been largely abandoned in recent years.
Gold allergy is common in patch-tested patients who have dermatitis; in one series, its rate approximated nickel allergy rates.[65] In another series of asymptomatic patients with gold restorations, 24 of 71 (33.8%) patients with gold restorations had a patch-test positive reaction to gold, as opposed to 7 of 65 (10.8%) "nongold" patients.[66] This highlights the need for assessment of the clinical relevance of gold patch test–positive results. Most individuals with hypersensitivity to gold (as confirmed by patch testing) are able to tolerate dental restorations that contain gold.[61,63,66–68]
Finally, dental restorations that contain nickel are associated with low rates of intraoral nickel-induced allergic reactions.[69–71] Removal of the dental implant has resulted in healing of the lesions within days or weeks in 49 to 95% of cases, which suggests a cause-effect relationship between the implant and the particular reaction.[69,72] Nevertheless, a few reports have shown that patients with a known nickel allergy as confirmed by patch testing do not develop oral complications in the setting of nickelcontaining dental restorations.[71,72] Oral tolerance may occur in subjects exposed to nickel from dental braces.[73]
Amalgam tattoos are another manifestation of mercury-related intraoral contact allergy.[72,74] They occur when small particles of dental amalgam get implanted into the oral soft tissues during dental procedures. Amalgam tattoos appear as blue, black, or gray asymptomatic patches on the oral mucosa. Burning mouth syndrome (BMS) and "burning lips syndrome" (a subtype of BMS) have been reported in association with strong allergy to cobalt, nickel, mercury, and gold.[58,59,75–80] Patients with BMS seem to have a higher frequency of contact allergy to gold than to mercury. In some cases, patients recovered completely after the removal of the mercury amalgam filling or dental gold. Also, an association between the use of DPT in bone cement and burning mouth reactions is seen in some patients.
Allergic Contact Dermatitis and In-stent Restenosis from Vascular and Cardiac Implants
Allergic contact dermatitis (ACD) and device failure may occur in response to implanted intravascular metal exposures. This topic was extensively reviewed recently by Honori and colleagues.[12]
Percutaneous transluminal coronary angioplasty and stent placement are becoming an increasingly common and effective method for the treatment of atherosclerotic disease. There are two main types of stents: bare metal stents and drug-eluting stents. Bare metal stents are composed of different alloys (typically with a backbone of stainless steel), which are the potential allergens for stent-induced ACD. It is thought that the composite metallic ions induce the expression of intercellular adhesion molecule 1 (ICAM-1) on endothelial cells. This in turn stimulates the recruitment of inflammatory cells and causes excessive neointimal hyperplasia. The proliferative neointimal response is responsible for intraluminal restenosis. Drug-eluting stents, on the other hand, are coated with polymers impregnated with a drug that inhibits intimal hyperplasia and subsequently yield a lower rate of DTH.
Nickel, chromate, manganese, and molybdenum eluted from stainless steel stents are, among the various metals, the most frequent allergens that induce ACD. Contact allergy to these metal ions is also thought to play a role in intraluminal restenosis. Table 9 [81–87] summarizes relevant studies evaluating restenosis. These studies cannot confirm a correlation between metal allergy and restenosis after initial stent implantation. At present, the exact relation between metal allergy and in-stent restenosis remains debatable.
Gold-coated stents were developed because gold (due to its higher stability) was thought to be less allergenic than the aforementioned metals. The frequency of contact allergy to gold was reported to be 5 to 10% in patch-tested patients with eczema.[88] Nevertheless, studies showed a higher risk of gold contact allergy in patients with goldplated endovascular stents.[89–92] Furthermore, the rate of restenosis was greater among patients with gold-plated stents than among those with stainless steel stents although this was statistically insignificant.[93,94] For these reasons, gold-plated stents are rarely used at this time.
Allergic reactions to patent foramen ovale (PFO) occluders have been reported, although rarely. The Amplatzer occluder (AGA Medical Corporation, Plymouth, MN), the only such device approved by the US Food and Drug Administration, is made of nitinol (approximately 45% nickel) and releases nickel; however, its effect on surrounding human tissue has not been studied. To date, only three patients have been reported to develop systemic allergic reactions to PFO occluders without apparent rash but with positive patch-test results.[95–97] Each of these patients' symptoms improved following either the removal of the device or the use of systemic corticosteroids.
Anecdotal reports of ACD from endovascular devices used for repair of an abdominal aortic aneurysm (AAA) are rare. Gimenez-Arnau and colleagues[98] reported the first case, that of a patient who developed generalized eczematous dermatitis 3 weeks after undergoing an AAA repair with a straight Vanguard endograft. This was thought to be due to the nickel contained in the endograft (the patient had a positive patch-test reaction to nickel). The patient responded well to systemic antihistamine and topical corticosteroid therapy.
The first implantable pacemakers were developed in the 1960s.[12] Pacemaker generators are made most frequently of titanium because of titanium's high biocompatibility. Other metals, including nickel and silicone, are also used, although in smaller amounts. The first case of pacemaker contact dermatitis was reported in 1970; since then, a growing number of cases have been documented.[99–107] Titanium is the most common allergen; nickel and silicone are other potential allergens. According to many reports, the use of a polytetrafluoroethylene (PTFE) sheet to wrap the device has been successful in preventing the recurrence of dermatitis during the reported follow-up periods of up to 3 years.[104,107–109] Recently, Ishii and colleagues[100] described the case of a 52-year-old man with Down syndrome who received a dual-chamber paced, dualchamber sensed, dual response rate moderated (DDDR) pacemaker for advanced atrioventricular block and developed cutaneous eczema and partial exposure of the generator 1 year after reimplantation. His patch-test result was positive for the metal of the generator (99.9% titanium) after 72 hours. The patient was subsequently reimplanted with a pacemaker wrapped with a PTFE sheet; 3 years later, the dermatitis had not recurred. Replacement with customized silicone or gold-coated pacemakers has also been reported to resolve pacemakerinduced allergic dermatitis; however, success rates are lower than those achieved with PTFE.[105,106,110]
Allergic Contact Dermatitis from Gynecologic Implants
Copper, nickel, and titanium are used in several devices for female contraception. Copper sulfate–containing intrauterine devices placed for temporary contraception are rare causes of systemic dermatitis. There are at least three cases of patch test–confirmed systemic ACD that resolved after the removal of a copper-containing intrauterine device.[111–113] A recent development in permanent contraception was the development of a nitinol-containing device for implantation in the fallopian tubes. This device (called Essure in the United States [Conceptus Incorporated, Mountain View, CA]) is implanted during an in-office transvaginal procedure into women desiring permanent contraception.[114] A contraindication to placement is previous nickel allergy. Likely, this contraindication is due to nickel release from the nitinol alloy, causing a potential systemic ACD. All prospective users of this device should be patch-tested with nickel prior to placement. The authors believe that nickel should not be used in such devices.
Dermatitis. 2011;22(2):65-79. © 2011 American Contact Dermatitis Society
Cite this: Cutaneous and Systemic Hypersensitivity Reactions to Metallic Implants - Medscape - Apr 01, 2011.










Comments