Immunity & Aging
In the recent decades, there has been an increase in the aging population across the world coupled with declining birth rates and an increased lifespan of individuals. The proportion of the elderly in developing and less developed countries is also expected to increase. According to the WHO, the proportion of old individuals (over the age of 60 years) will rise to 22% of the world population.[101] In light of this, it is becoming critical to improve our understanding of aging and its associated diseases to support existing healthcare systems coping with the burden of diseases and to promote healthy aging.
Diseases are a concern for the aging population as the recovery is longer, and most of the time incomplete, often leading to hospitalization, complication, integration in specialized care centers and ultimately death. The higher incidence of infectious diseases (e.g., pneumonia and influenza), autoimmunity (e.g., rheumatoid arthritis), cancer (e.g., prostate and lung), Type 2 diabetes and cardiovascular diseases is an obvious issue for the elderly. The link between these diseases is immunity, and loss of immune functions may explain the age-associated incidence of such diseases by reduced immunosurveillance, polyfunctionality or regulation.
The name 'immunosenescence' has been used to describe loss of immune functions in elderly individuals (>65 years old). Although the mechanisms leading to immunosenescence are not clear, it has been associated with increased susceptibility to diseases, infections and poor response to treatments and vaccination.[1] With advancing age, antigen-specific immunity is eroded partly due to alterations in the innate immunity. The changes affecting the immune system are leading to global dysfunctions. Although immunosenescence is characterized by changes in both the innate and adaptive arms of the immune system,[2] the important contributor to decline in immune function in the elderly is the changes observed in adaptive immunity, including T and B lymphocytes. Antigen encounter normally induces an immune response to eliminate the invader by the concerted T- and B-cell responses, which enables a faster and stronger response following secondary encounter (immune memory).
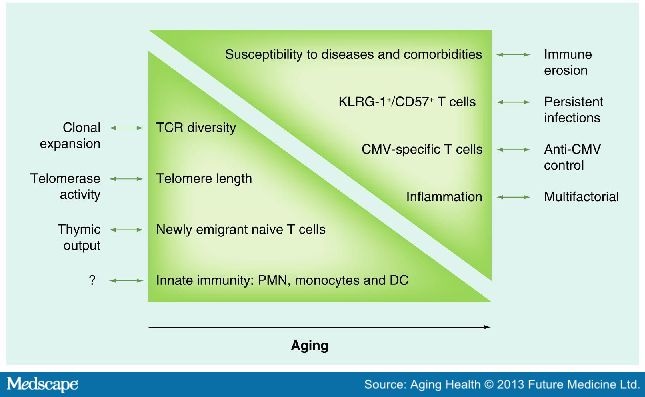
With aging, the ability to maintain receptor diversity reduces,[3] and this is paralleled by the reduction in the number of naive T cells in the periphery.[4] One hypothesis put forward to explain this phenomenon is the involution of the thymus, where T-cell maturation happens (Figure 1). Thymic involution is not only observed in the elderly, but begins much earlier in life, after puberty. With age, the number and frequency of circulating naive T cells (CD45RA+CCR7+CD28+CD27+) is reduced, and this is associated to reduced thymopoiesis, as well as increased number of memory cells due to pathogens encountered during the course of life.[5] Another hypothesis to, at least partly, explain immunosenescence is the telomere length. Telomeric repeats (TTAGGG) are present at the end of chromosomes and serve to protect DNA from alterations (Figure 1). An intense replicative history will thus induce a shortening of telomeres, which put these cells at risk for DNA mismatch.[6] Thus, telomere shortening has been primarily identified in highly differentiated effector memory CD8+ T cells, most of which are CD28− T cells.[7] As loss of telomeric repeats is associated with loss of proliferative capacity, telomeres have been associated to replicative senescence. It is of note that despite this, evidence shows that telomere length is not the shortest in the highly differentiated T cells,[8] suggesting a more complex relationship between telomeres and senescence.
Figure 1.
The components of immunosenescence. The age-associated changes in immune phenotypes are depicted. Represented on the left side are items that show decline with age, while items on the right show items that demonstrate upregulation with age.
?: Unknown cause; CMV: Cytomegalovirus; DC: Dendritic cell; PMN: Polymorphonuclear neutrophil; TCR: T-cell receptor.
Aging Health. 2013;9(1):35-47. © 2013 Future Medicine Ltd.