One of the greatest medical threats of our time is the impending one of antibiotic-resistant bacteria.
Slowly but surely over decades of antibiotic use, the bugs we fight with drugs have evolved to become resistant to those drugs. This is a huge—and growing—problem around the globe, with many bacterial infections rapidly becoming resistant to our arsenal of defenses.
One solution to the problem may be to use an unlikely ally: viruses.
The bacteria that cause conditions ranging from pneumonia to tuberculosis, blood poisoning and gonorrhea are gaining rapid resistance to the antibiotics we used to use against them, according to the World Health Organization. Nearly all strains of Staphylococcus aureus are resistant to penicillin in the U.S., with many developing resistance to methicillin, causing MRSA (or methicillin-resistant Staphylococcus aureus), infections.
"Superbugs are bacteria that have evolved the ability to withstand [or become resistant to] antibiotics and other drugs we use to treat infections. We call this phenomena antimicrobial resistance, or AMR for short," Jeremy Barr, a senior lecturer in environmental microbiology at Australia's Monash University, told Newsweek.
"AMR is a natural process, and in fact we have found antibiotic-resistant bacteria from samples that are hundreds of thousands of years old," he continued. "With respect to superbugs, our collective overuse and reliance on antibiotics has bred or selected for bacteria that are increasingly becoming resistant to these drugs. Put simply, the more we use an antibiotic, the faster we will select for superbugs."
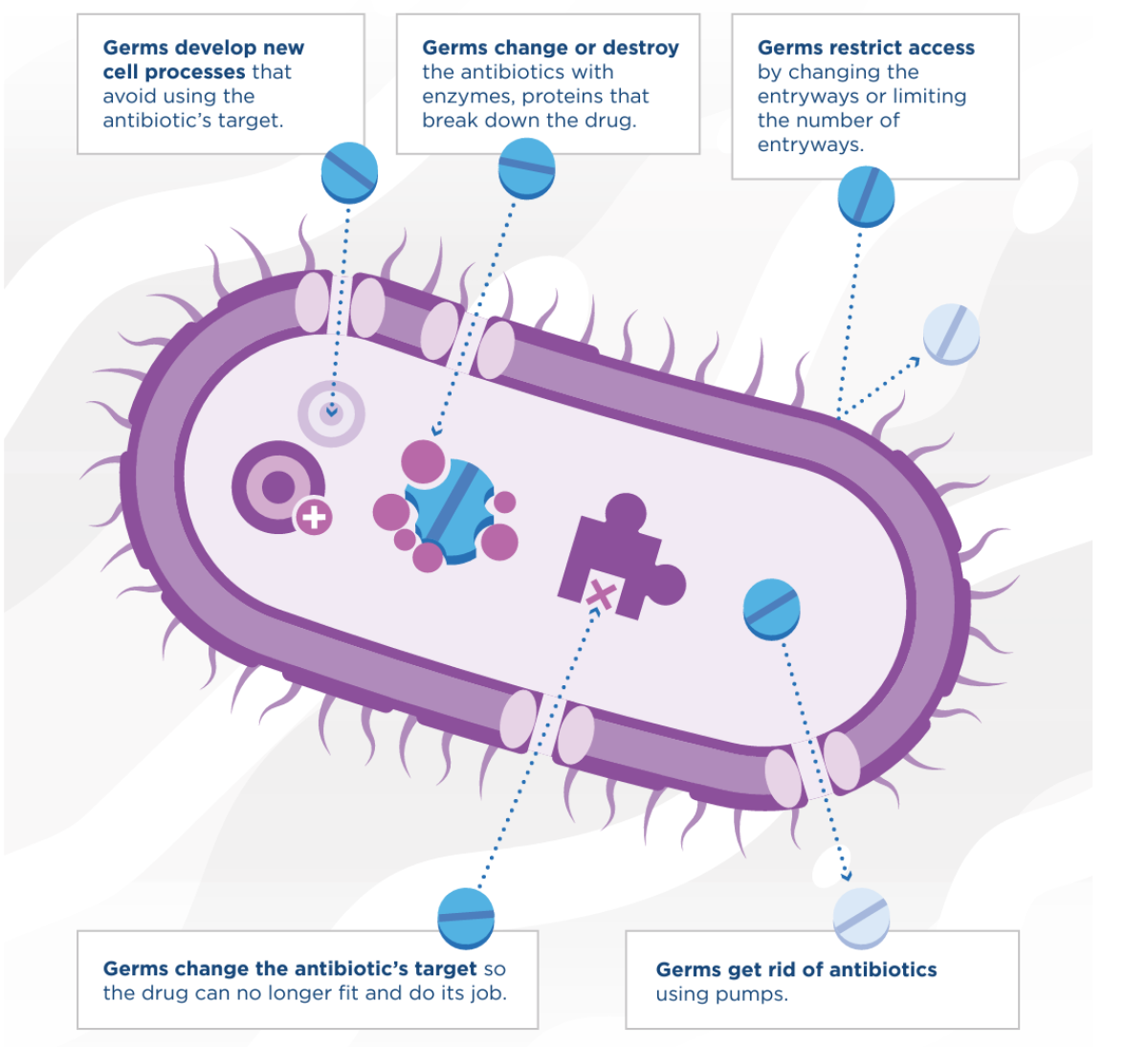
The Centers for Disease Control and Prevention says that in the U.S. over 2.8 million antimicrobial-resistant infections occur each year, resulting in 35,000 deaths. Antibiotic resistance is forecast to cause 10 million worldwide deaths each year by 2050.
"There are many instances of certain superbugs that are now resistant to all of our clinically relevant antibiotics," Barr said. "We are now having to go back and use old antibiotics as last resort options—or using other drugs that have toxicity concerns—as none of our other drugs are working to fight these infections."
So what can we do to stem the tide of bacterial infections that are becoming increasingly resistant to our treatments for them? Many scientists hope that viruses that kill bacteria—called bacteriophages—may be the answer. In fact, bacteriophages are one of the most abundant and diverse organisms on the planet.
"There are more phages on the planet than stars in the universe," Barr said. "There are more phages in your body than there are of your own human cells."
Because phages are natural predators of bacteria, we can use them to fight bacterial infections.
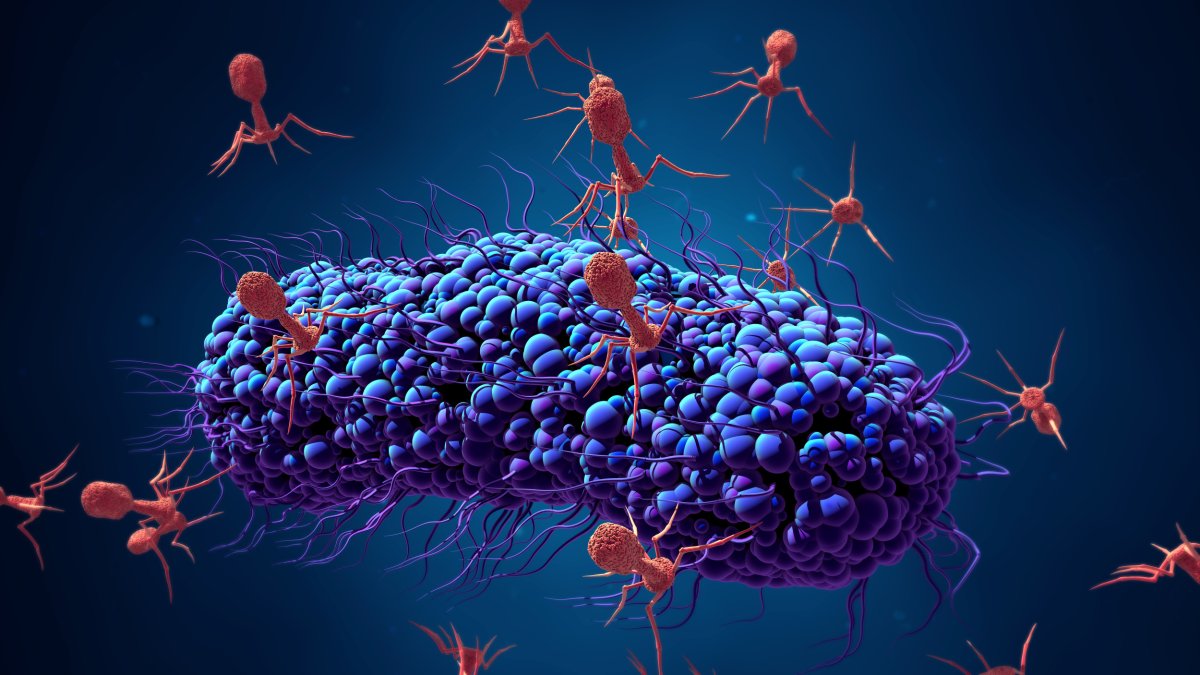
"They provide alternatives that can be used alone or in combination with traditional antibiotics," Andrew Millard, an associate professor of genetics and genome biology at the U.K.'s University of Leicester, told Newsweek.
He continued: "They have both pros and cons. Phages are often specific to just one type of bacteria, so they don't kill all the natural gut flora like antibiotics do. It's like carpet bombing with antibiotics or precision strikes with phages. They target only the bacteria that are causing the disease."
However, one of the biggest limitations, but also benefits, of using phages is that they are very specific to the type of bacteria that they target.
"Unlike antibiotics, which can kill many different types of bacteria at once, phages are very specific for the bacteria they infect and kill," Barr said. "This means that to use phages to treat a patient's infection, which is called phage therapy, we first need to find a phage that can kill that patient's bacterial infection.
"The benefit here is that phages are nearly unlimited, so we likely will not run out of them like we have done with antibiotics, and as they are so specific they do not cause side effects such as affecting your gut microbiome like antibiotics do," he said.
These phages can also be used in other areas to replace antibiotics, such as in agriculture.
"Antibiotics [are] being widely used in agriculture and accounting for a large amount of antibiotic production," Barr said. "With this use of antibiotics in agriculture, AMR resistance occurs, which can then be transferred to bacteria that infect humans. So by reducing in other areas than phage therapy in humans, it can help with the superbug problem in an approach known as One Health."
#ThursdayThoughts: #Antibiotic resistance is a major #publichealth concern in our country. In 2017 alone, @CDC_NCEZID tracked more than 200 cases of Candida auris in the U.S. https://t.co/1NpxOUH7nK pic.twitter.com/kCy0KxrK8J
— CDC Emergency (@CDCemergency) April 12, 2018
While phage therapy may seem like a perfect cure, there is one problem: Bacteria can develop resistance to phages too.
"Bacteria have multiple ways to combat bacteriophages and be resistant to them," Barr explained. "This is an entirely natural process that has been occurring for millions of years. The difference is phages have also been evolving at the same time to overcome the resistance that bacteria are evolving. It is a never-ending evolutionary arms race. So yes, bacteria will become resistant to a phage."
However, all is not lost, as we can mitigate the likelihood of this occurring.
"Using combinations of phages—phage cocktails—can help prevent this, so bacteria can become resistant but not resistant to all phages," Millard said. "There are lots of different types of phages, so putting different combinations together can help prevent resistance from occurring. Developing the right cocktail of phages is one of the things that is being developed."
Additionally, because of the way phages kill their bacteria, we see that phage-resistant bacteria are less able to cause infection and disease.
"[This] makes them easier to treat and clear from patients," Barr said.

In recent years, there has been a lot of research and progress in phage therapy.
"A recent study from last year calculated that more than 2,500 patients had received phage therapy to date, with over 70 percent of them showing improvement in their infections," Barr said. "Phages are very safe to use and are very effective against superbug infection."
This nascent treatment is growing in usage in many countries.
"Recently, they have been used in the U.K. in Scotland to treat diabetic foot ulcers," Millard said. "Also used in the U.S. in emergency cases with some success—it is not a standard treatment as of yet. The U.K. has a very strong base in phage research. Clinical trials have now been funded in the U.S. for using phage therapy in cystic fibrosis. "In the U.K., the first Center of Phage Research has been created at the University of Leicester."
Barr said that Georgia and Poland, followed by Russia, have the longest history of treating patients with phages. Some of these institutes have treated patients for over 70 years and continue to do so today.
"However, the research and understanding of how phages work and conducting proper clinical trials has not yet eventuated," he said. "As such, many other countries from around the world, including many countries in Europe, the U.S., Australia, China and others, are furthering this work," Barr said.
In other nations, phage therapy is struggling to get off the ground because of issues with regulation.
"We need more research funding to ensure phage therapy continues to be safe and can be provided to more patients where needed," Barr said. "There are still many issues around safety and regulation by the FDA, which as a result means that phage therapy is still experimental and not widely available.
"Here at Monash University, we have built the Monash Phage Foundry, which is a facility where we can produce phages to treat local patients suffering life-threatening superbug infections," he said. "I hope we will see effective and accessible phage therapy being offered in several countries within the next five years."
Do you have a tip on a science story that Newsweek should be covering? Do you have a question about phage therapy? Let us know via science@newsweek.com.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Jess Thomson is a Newsweek Science Reporter based in London UK. Her focus is reporting on science, technology and healthcare. ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.








