Results
All 26 patients who were enrolled completed the study. There were 18 females and 8 males in the study, and 1 person was less than 18 years of age. Eighteen subjects (69%) had at least one positive reaction that was interpreted as being allergic, reaction to thimerosal being the most frequent allergic reaction noted ( Table 2 ). No subjects had a positive reaction to the OTC tapes and bandages in their whole form that was interpreted as being allergic or had a known relevant allergic reaction to a chemical on our custom adhesive tray. (There were study subjects who had positive allergic reactions to chemicals on our custom adhesive tray, but after contacting the adhesive manufacturer of the specific product in question, we were informed that the chemical was not present in the tape or bandage to which the subject noted a reaction. We had to rely on phone conversations and E-mail correspondence for this information, as we were never provided a written ingredient list of any chemical constituents of the bandages or tapes by any of the manufacturers, despite concerted efforts.)
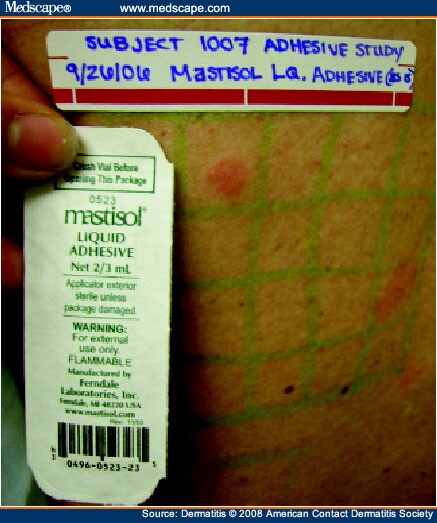
Four subjects (15%) had clinically relevant allergic reactions. The first subject had an allergic reaction to Mastisol liquid adhesive (Ferndale Laboratories, Ferndale, MI) (Fig 5), which by history was applied under Steri-Strips (3M, St. Paul, MN) covering a biopsy site, which developed a pruritic bullous reaction. The second and third subjects had positive reactions to neomycin and bacitracin, both of which were applied under Kendall adhesive bandages following biopsies and resulted in pruritic plaques extending beyond the bandage. The fourth patient had an allergic reaction to Cortisone-10 (Pfizer, New York, NY) cream, which she applied under a Band-Aid (Johnson & Johnson, New Brunswick, NJ) and subsequently developed ACD. At 48 hours, 2 of the 26 subjects (8%) had a probable irritant reaction to at least one OTC tape or bandage in its whole form when it had been left on for 48 hours; 8 of 11 subjects (73%) had an irritant reaction to at least one OTC tape or bandage in its whole form after it had been left on for 7 days (see Figs 2 and 3). Of these 8 subjects who had an irritant reaction, 2 had a positive reaction at 48 hours. In the eight study subjects who had probable irritant reactions to various bandages, Curad Adhesive Sheer Bandages (Beiersdorf, Hamburg, Germany) were always included among the bandages to which they had such reactions. No patients demonstrated dermatographism. There were no reactions, irritant or allergic, to the Scanpor tape (which was left on all of the subjects for 7 days). There was no correlation between a history of atopy and irritant or allergic patch-test reactions.
Day 7 reading, showing allergic contact dermatitis from Mastisol, the chemical causing the patient's reaction at the site of the medical adhesive application.
Dermatitis. 2008;19(1):32-37. © 2008 American Contact Dermatitis Society
Cite this: Allergic Contact Dermatitis from Medical Adhesive Bandages in Patients Who Report Having a Reaction to Medical Bandages - Medscape - Feb 01, 2008.








Comments