Abstract and Introduction
Choroidal neovascularization (CNV) is one of the most important vision-threatening complications secondary to pathological myopia (PM). In the last 20 years, there have been rapid advances in the treatment options for myopic CNV. The aim of this article is to give an overview of several modalities of treatment of CNV in myopes, including laser photocoagulation, transpupillary thermotherapy, radiotherapy, photodynamic therapy with verteporfin and surgical treatments. Based on the scientific progress achieved in the past few years, it is possible that patients with myopic macular degeneration complicated by CNV may benefit from a new ophthalmic frontier of pharmacologic therapy, including intravitreal injections of anti-VEGF drugs. The current status of these novel therapeutic agents is also discussed.
Pathological myopia (PM) is one of the leading causes of visual disability in the world from 20-50 years of age.[1] Prevalence rates of PM vary between 2 and 9%, depending on the race and age of the population. The prevalence of myopia is especially high in parts of East Asia, with rates of 9-21%, compared with 2-4% in Caucasians. It is still unknown whether this high prevalence reflects primarily genetic factors or a more complex response to environmental stimulation occurring on a susceptible genetic/ethnic background.[2] Visual acuity (VA) decreases with severity of myopic refractive error, particularly for myopia higher than 3 diopters. Myopia was a predictor for visual impairment (odds ratio [OR]: 1.26) and for blindness (OR: 1.20) after 5 years. There is also an association between severe myopia and myopic macular degeneration.[3,4]
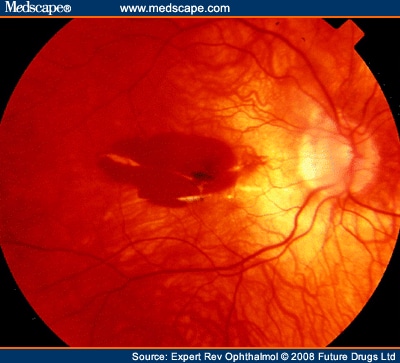
The pathogenetic mechanisms of myopia are still not completely elucidated, although genetic and enviromental factors may both play a role. PM is characterized by excessive axial length (≥26 mm), with secondary degenerative changes of the sclera, choroid, Bruch's membrane, retinal pigment epithelium (RPE) and retina. These changes are concentrated in the midperipheral fundus and in the macular area. The most common posterior pole changes include posterior staphyloma, optic nerve crescents, chorioretinal atrophy, lacquer cracks and the development of choroidal neovascularization (CNV) in the center of the macula via VEGF modulation (Figure 1 and Figure 2).[5,6]
Fundus photography shows choroidal vessels under a thin retinal pigment epithelium.
A macular hemorrhage is visible associated with some lacquer cracks.
Other authors have already discussed the management of CNV in myopic macular degeneration and this article is an up-to-date review of this disease, considering the new therapeutic options from the last few years.[7,8]
CNV is one of the most important vision-threatening complications of PM and occurs in 5-10% of myopic patients. Myopic CNV is predominant in females (67%) who may reflect estrogen receptor expression in CNV and external influence of estrogen.[9] The development of CNV is subfoveal in approximately 58% of cases and juxtafoveal in 32%.[2] Choroidal neovascularization usually affects young adults of 40-50 years of age, and has a poor prognosis with a significant risk of visual deterioration.[10,11] Linear breaks in Bruch's membrane secondary to stretching and degeneration of the choroid make these eyes at risk for CNV. These breaks often appear clinically as lacquer cracks and are associated with CNV in 82% of eyes. It is unclear, however, what causes the growth of new vessels through the breaks in Bruch's membrane.[12]
In its advanced stage, CNV appears as a Fuchs' spot which is a macular scar with pigment clumping and, sometimes, hyperpigmentation in association with fibrosis and retinal atrophy is present.[13] Among myopic patients with pre-existing CNV, more than 30% will develop CNV in the fellow eye within 8 years.[11]
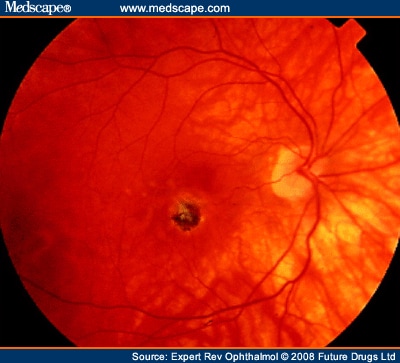
The slit-lamp binocular macular biomicroscopy demonstrates that CNV appears as a small pigmented or red lump beneath the retina and is often surrounded by a dark pigment ring with macular hemorrhage (Figure 3). Typically, myopic CNV is located between the neurosensory retina and the retinal pigment epithelial layer.[13] The sensory retinal elevation associated with this CNV seems to be extremely small, shallow and difficult to distinguish clinically owing to the minimal accumulation of subretinal fluid and exudation.
Fundus photography shows a pigmented choroidal neovascularization in macular region.
Expert Rev Ophthalmol. 2008;3(3):311-323. © 2008 Expert Reviews Ltd.
Cite this: Management of Choroidal Neovascularization in Myopic Macular Degeneration - Medscape - Jun 01, 2008.











Comments