Types of VAD
VADs are usually classified according to the mode of blood flow, namely pulsatile or continuous. Continuous types can be further subdivided based on the biomechanics of the pump: centrifugal or axial. Some continuous VADs can create pulsatile waveforms by periodically changing the pump speed. VADs are also classified based on the location of a pump relative to the patient: intracorporeal, paracorporeal, and extracorporeal. In general, a pulsatile VAD is paracorporeal, and an axial VAD is intracorporeal. A centrifugal pump is used in multiple settings. Selected examples of each category will be reviewed in the following section.
Pulsatile Pumps
Two pulsatile systems have proved successful in supporting children of all ages, from the newborn to the adolescent. The most widely used is the Berlin Heart EXCOR (Berlin Heart AG) and less frequently used is the Medos HIA (Medos Medizintechnik AG, Stolberg, Germany). Both systems are paracorporeal and consist of a pneumatic pulsatile compressor-operated diaphragm pump with valves, having a transparent polyurethane pump housing that allows early thrombus detection and fast and safe pump changes if necessary.
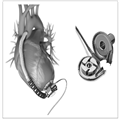
Berlin Heart Excor. These blood pumps are available in five different sizes with stroke volumes of 10, 25, 30, 50, and 60 mL. The smallest pumps are suitable for neonates and infants weighing from 3 to 8 kg and the 25- and 30-mL pumps for children weighing up to 20–25 kg. Special long-term silicone cannulas connect the blood pumps to the body. For left-heart support, cannulation is preferred in the apex of the left ventricle, as this provides better unloading than the alternative left atrial cannulation site. In addition, there has been anecdotal experience that apical cannulation is associated with fewer thrombotic complications than atrial cannulation, but this is not well documented in the literature. For right-heart support, the cannulas are inserted into the right atrium or the right ventricular apex (depending on the size and trabecularization of the myocardium) and to the pulmonary artery. The cannulas are designed to exit the body through the upper abdominal wall. A Dacron velvet cover in the middle portion of the cannula promotes rapid growth and fixation of tissue as a biological barrier against ascending infections (Fig. 1).
Figure 1.
Berlin Heart EXCOR. Reprinted with permission from Berlin Heart, The Woodlands, TX.
Valves are located at the inlet and outlet positions of the blood pump connection branches, thus ensuring unidirectional blood flow. Pulse rate, systolic drive pressure, diastolic suction pressure, and the relative systolic duration can all be monitored and adjusted on the IKUS in-hospital driving unit. The IKUS operates pumps of any size in univentricular or biventricular configuration. A mobile driving unit is available for larger pumps (50 and 60 mL), but the mobile system is currently available only in Europe. All blood-contacting surfaces are coated with heparin (Carmeda, Upplands Väsby, Sweden), providing early protection against thrombosis.
The Berlin EXCOR enables long-term support, allowing for end-organ recovery and facilitating extubation and mobilization, all of which are important to raise the likelihood of successful transplantation. European experience suggests the EXCOR provides stable circulatory long-term support for up to 2 years. The device has provided safe outcomes even in newborns weighing 3 kg.[10]
Long-term support with the EXCOR VAD is clearly superior compared with ECMO support. The use of the EXCOR is associated with less blood loss and fewer blood products.[11,12] The main differences between the two modes of MCS were recently highlighted in the recent FDA IDE trial.[7] In this prospective multicenter single-group cohort study, 48 children with EXCOR were compared with a historical control group of children who received ECMO support. In the smaller patient cohort (median age and weight of 1 yr and 9 kg), 88% of patients achieved good outcomes (either bridge-to-transplant or bridge-to-recovery) at a median support duration of 28 days. In contrast, the median time to death or weaning from the device with an unacceptable neurologic outcome in the matched ECMO group was 13 days.
Axial Pumps
There are several major advantages of continuous pumps compared with pulsatile pumps. Clinically important advantages include smaller size, valve-free structure, lower energy consumption, and reduced cost.[13] The priming volume of typical axial pumps is 10 ± 5 mL, which is even smaller than that of centrifugal pumps (25 ± 10 mL). An axial pump generates flow by rotating its internal impeller based on the principle of an Archimedes screw. At the cost of its small size, the impeller has to be operated at a higher revolution (typically >8,000 rpm) compared with that of centrifugal pumps (typically <3,000 rpm). This feature has important clinical implications in terms of device durability and hemolysis, especially when the support duration is long.
DeBakey VAD/HeartAssist 5. The DeBakey VAD Child (MicroMed Technology, Houston, TX) is a device approved by the FDA in 2004. This device is a miniaturized version of the adult DeBakey VAD, intended to be used for larger children with BSA of greater than 0.7 m2. The DeBakey VAD Child, however, has had limited clinical application because of a high incidence of thromboembolic events.[14] In an attempt to reduce this important morbidity, the newer version of this VAD, the HeartAssist 5, has a redesigned inducer/impeller to improve its blood-handling characteristics. The IDE Clinical Study for the HeartAssist 5 is currently being planned;[15] it will be a prospective randomized multicenter clinical trial to evaluate the safety and efficacy of the HeartAssist 5 compared with the HeartMate II for bridge-to-transplantation.
HeartMate II. The Heart- Mate II (Thoratec, Pleasanton, CA) is the most widely used implantable device, with more than 10,000 implants worldwide (as of April 2012). The HeartMate II is currently the only continuous-flow left-ventricular assist device (LVAD) approved by the FDA for both bridge-to-transplantation and destination therapy. Because of its relatively small size, the HeartMate II can be implanted in older children or adolescents with body weight of greater than 45 kg or BSA of greater than 1.3 m2. The inflow cannula is directly inserted into the LV apex, and the outflow graft is connected to the ascending aorta. The pump is placed in a "pump pocket" that needs to be surgically created by dissecting the diaphragm free from the abdominal wall. The pump is connected through a driveline with the controller and two batteries outside of the body (Fig. 2).
Figure 2.
HeartMate II. Reprinted with the permission of Thoratec Corporation, Pleasanton, CA.
Initial clinical results from the adult HeartMate II multicenter pivotal clinical trial for bridge-to-transplant conducted from 2005 to 2008 reported survival rates of 75% at 6 months and 68% at 1 year.[16] After the FDA approved the HeartMate II for bridge-to-transplantation, a study comparing the Heartmate II to other types of VAD found that adverse event rates in adults were similar or lower to the comparison group for all events. Bleeding was the most frequent adverse event in both groups (1.44 vs 1.79 events/patient-year). Operative 30-day mortality for HeartMate II was 4% versus 11% for the controls.[17]
Although a large number of studies are available in the adult population, very little is known regarding outcomes of the HeartMate II in children. Most of the available pediatric literature is limited to sporadic case reports, except for the study done by Cabrera et al.[18] Using the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), outcomes of pediatric patients (n = 26, <18 yr old) with HeartMate II were compared with those of young adults (n = 319, 19–39 yr old). At 6-month follow-up, survival to transplantation, ongoing support, or recovery was 95% for the pediatric group, which was equivalent with the young adult group (96%, p = 0.341). This study suggests the HeartMate II is an effective tool for larger children with endstage heart failure.
Centrifugal Pumps
One of the applications of centrifugal pumps for pediatric VAD is short-term support with systems such as the CentriMag or PediMag (Thoratec). The short-term VAD system is essentially similar to ECMO systems, with the major difference being the absence of an oxygenator. Advantages of the short-term VAD system compared with ECMO include lower priming volumes, adequate left (or systemic) ventricular decompression, lower heparin requirements, less hemolysis, ease of transport, and relatively low costs.[19,20] On the other hand, shortterm VAD support generally requires central cannulation, except for a specially designed percutaneous cannula that allows left atrial cannulation through the atrial septum.[4] Even though short-term VAD support using an extracorporeal centrifugal pump has been gaining widespread application in the adult population, there are only a few series available in children.[21–23] This is because ECMO still remains the mainstay of short-term mechanical circulatory support in the majority of pediatric heart centers worldwide.
Another application of centrifugal pumps is an intracorporeal VAD system. As discussed earlier, one of the important advantages of centrifugal pumps over axial-flow pumps is that centrifugal pumps run at significantly lower rpm, which is less likely to cause blood trauma. This feature is particularly important with long-term support and why the newer generation of intracorporeal VADs (e.g., HeartWare, DuraHeart, Levacor, Evaheart, etc) employ a centrifugal design.
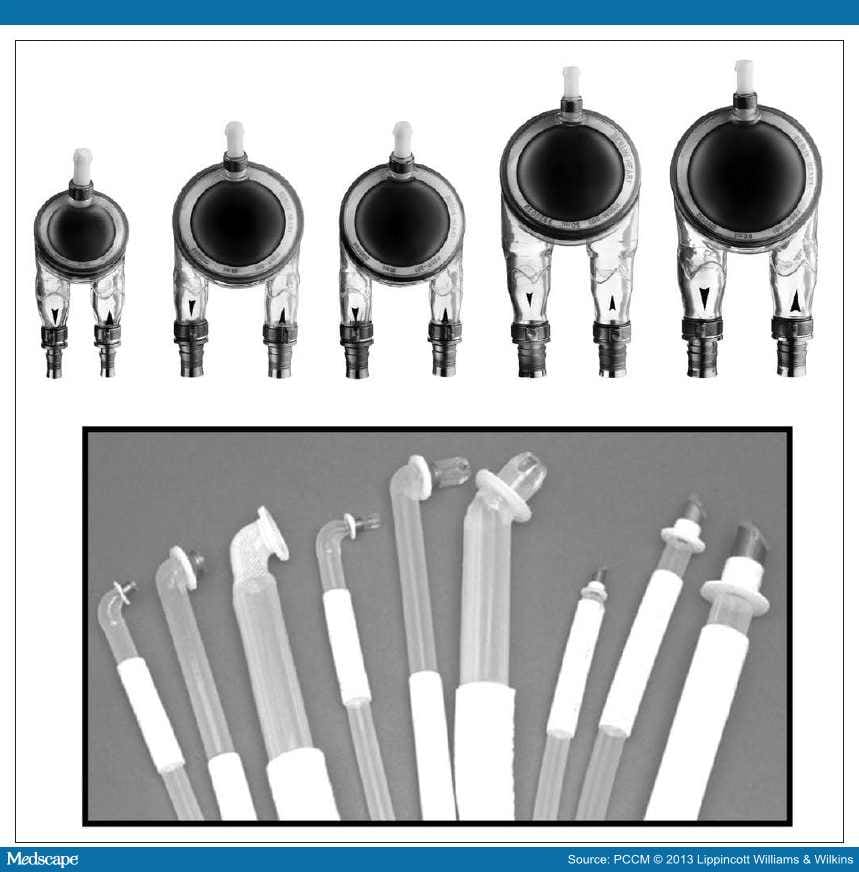
Levitronix CentriMag and PediMag. The CentriMag system is a continuous-flow device applicable for left, right and biventricular support in adults and children.[20,24] The PediMag is a miniaturized version of the CentriMag, intended to be used for patients weighing 10 kg or less. The key feature of these pumps is their bearing-less magnetically levitated impeller with two magnetic forces, one as a passive magnetic force raising the impeller and the second an active magnetic force created by an electrical current in the motor centers, spinning the impeller and causing forward flow. The blood enters the pump from the 3/8-inch inlet (1/4 inch with PediMag) on the top of the pump, is propelled by the impeller in a circular motion, and exits the pump on the side with a 33 cc (14 cc with PediMag) priming volume. The CentriMag can flow up to 10 L/min whereas the flow range for the PediMag is 0.4 to 1.7 L/min. These devices are generally used for short- or intermediate- term support mainly as a bridge-to-decision or bridge-tobridge with the possibility of changing to a longer term VAD[25] but occasionally as a bridge-to-transplant (Fig. 3).[26]
Figure 3.
PediMag. Reprinted with the permission of Thoratec Corporation, Pleasanton, CA.
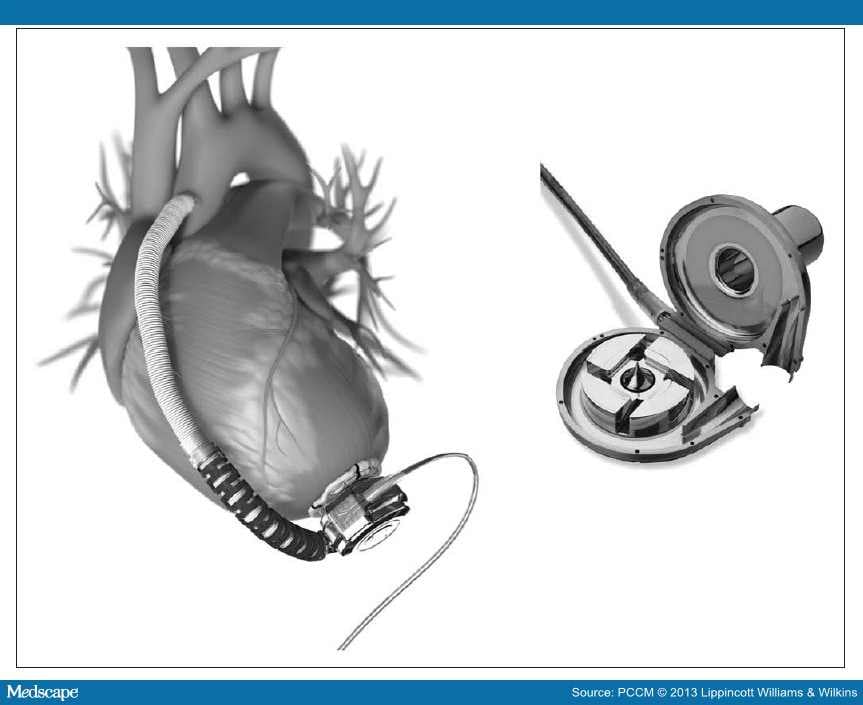
HeartWare HVAD. The HVAD (HeartWare, Framingham, MA) is one of the newest devices with the advantage of being smaller than previous LVADs. The pump weighs only 140 g and offers full-output circulatory support with up to 10 L/min flow. It is implanted in the left ventricle and connected to the ascending aorta and is a centrifugal continuous-flow pump with a hybrid magnetic bearing. It is implanted within the pericardial space and connected to the external power and control panel via the driveline that is externalized through the abdominal wall.[27] Recently, centers have also reported the use of the HeartWare devices as a biventricular-assist device in adults (Fig. 4).[28]
Figure 4.
HVAD. Reprinted with the permission of HeartWare, Framingham, MA.
The HeartWare has received CE Marking for the system in the European Union and Therapeutic Goods Administration approval in Australia. On November 20, 2012, the FDA approved the HeartWare LVAD for the purpose of bridge-to-transplant based on data from a clinical trial known as the ADVANCE trial.[29] The trial compared outcomes from 137 advanced heart failure participants using the HeartWare system with outcomes from similar patients in the INTERMACS.[30] This FDA approval was unusual in that they approved an LVAD using registry data as a control. The INTERMACS, established in 2005 as a joint effort involving the FDA, NHLBI, Centers for Medicare and Medicaid Services, clinicians, scientists, and industry, collects information on patients implanted with approved mechanical circulatory support devices. The pediatric version of INTERMACS, called PediMACS, was launched in September 2012.[31]
The group from Berlin reported their first experience with this assist system in the pediatric population. Seven children (6–16 yr old) received it as a bridge-to-transplantation. Six patients have been successfully bridged to transplantation. Median support time was 75 days. One child was still on continuous mechanical support. None of the patients suffered a thromboembolic event or an infection.[32]
Deltastream DP3. The Deltastream DP3 (Medos Medizintechnik AG, Stolberg, Germany) is a newly designed rotational pump with a diagonally streamed impeller. It has a low priming volume of only 16 mL and can be used in children of all ages, with optional operating methods producing pulsatility with 40–90 beats/min (flow 0–8 L/min, speed 100–10,000 rpm, weight 38 g). The pump's compact design allows mobility of the whole system and flexible positioning of the pump system and a reduction in tubing length, with consequent reduction in filling volumes and less blood contact with foreign surfaces. Several control systems offer safety functions: The preload control prevents aspiration of the cannula, and the zero-flow mode allows reduction or even a brief interruption of the flow without backflow. Its bubble detectors and pressure sensors make the pump reliable (Fig. 5).
Figure 5.
Deltastream DP3. Reprinted with the permission of Medos Medizintechnik AG, Stolberg, Germany.
Pediatr Crit Care Med. 2013;14(5):S20-S26. © 2013 Lippincott Williams & Wilkins