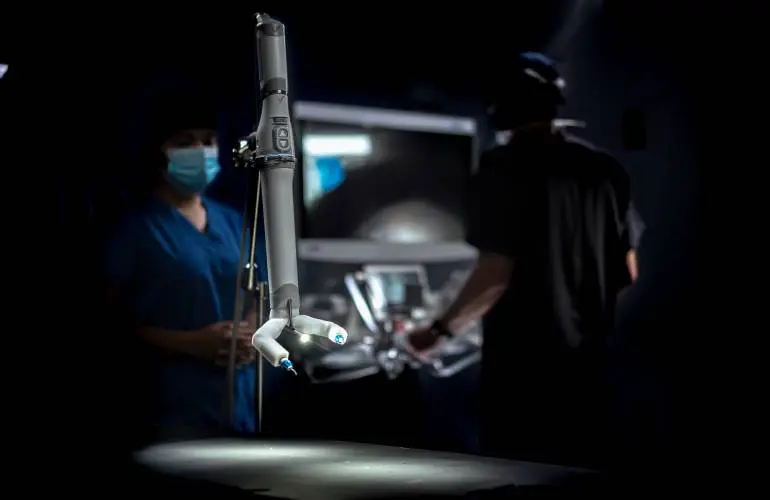
After nearly two decades of work, Nebraska-based Virtual Incision has received U.S. Food and Drug Administration (FDA) authorization for the world’s first Miniaturized Robotic-Assisted Surgery system, called MIRA.
Virtual Incision’s surgical robot, MIRA, will be used to operate on adults undergoing colectomy procedures, in which part of the colon is removed. Company leaders hope to expand the robot’s application to other types of surgical procedures in the future.
Virtual Incision was founded in 2006 by University of Nebraska-Lincoln (UNL) professor Shane Farritor and University of Nebraska Medical Center (UNMC) professor Dmitry Oleynikov. Farritor’s background is in mechanical engineering and Oleynikovs’ background is in minimally invasive surgery.
From Collaboration to Commercialization
Michael Dixon, president and CEO of UNeMed—the technology transfer and commercialization office at UNMC—has worked closely with Farritor and Oleynikov throughout the development and approval of MIRA.
“We often say that the best inventions come at the intersection of science,” said Dixon. “The fact that we had really good clinical, medical and surgical expertise, paired with the engineering piece, allowed this device to be brought to life.”
“That collaboration is critical. If it was either one of them designing on their own, we probably wouldn’t have gotten to this point,” Dixon added.
In addition to the complementary expertise of the founders, collaboration between UNL and UNMC was essential in the development of this new medical technology. Mechanical engineering of MIRA took place at UNL, while surgical logistics were examined and developed at UNMC.
MIRA was developed as the world’s first Miniaturized Robotic-Assisted Surgery system in an attempt to create an accessible, minimally invasive, small-scale robot that could change the future of medicine.
FDA authorization serves as a substantial milestone in the journey toward product finalization. “Biomedical companies face a really big challenge…because you can’t just go sell things and get feedback,” according to Dixon.
Strict regulations on medical testing have left Farritor and Oleynikov asking questions and seeking answers for years. Now the company will begin making sales and soliciting constructive product feedback so that improvements can be made.
The development of MIRA is complex and requires significant funding from investors like Bluestem Capital, Endeavour Visions and InVivium Capital. So far, this investment has been met with rewarding advancement, but little financial return.
“It [FDA authorization] will actually allow them to start bringing in revenue from sales which will be a big deal,” says Dixon.
Getting to this point has required multiple rounds of funding to continue project development. Initial funding was secured in 2010, allotting $2M for research and development. This was followed by a second $11.2M-round of funding in 2015, and a third in 2018 for $18M. Subsequent rounds in recent years have brought the total investment in the company to $137M.
Beyond a financial impact, FDA approval requires the presentation of extensive clinical research and review. Dixon said the FDA review will be an official stamp of approval demonstrating that the product is safe and effective.
Small Stature: Big Potential
The MIRA device can fit in a briefcase making it extremely portable. That means it’s easier for smaller hospitals to adopt the new technology without consideration for operating room space constraints.
“It is important for hospitals to make sure that they are using their rooms to the maximum capacity, so this smaller, portable robot will give smaller hospitals a cheaper device… they can move room to room,” Dixon said.
With FDA approval, the MIRA device will begin to perform colectomy procedures, a surgery used to treat colon cancer, on adult patients. Before the use of this advanced technology, a colectomy was performed by creating a long, vertical incision across the stomach. Now, with the use of MIRA, only a small hole will be created, significantly reducing recovery time. Smaller incisions result in fewer stitches.
Procedures performed with MIRA will reduce recovery time in the hospital to around two days, down from seven days previously.
More Accessible Solutions
Virtual Incision plans to continue development and expand the use of MIRA beyond colectomy procedures. Currently, there is no timeline for advancement into other surgical procedures, but Virtual Incision wants to integrate MIRA into simple medical operations soon.
Significant testing must be completed to determine the viability of different procedures. To move forward, MIRA needs to be quicker and more efficient than alternative options.
“When moving on to other procedures, proof of concept is really important,” Dixon said.
Beyond advancement into other procedures, Virtual Incision hopes that MIRA will result in more accessible healthcare worldwide. The portability of the device will allow it to be transported to war zones, rural communities and areas impacted by natural disasters.
“You can have one of these devices in a small community hospital and have it run by an incredibly experienced surgeon at a big primary hospital in Detroit,” according to Dixon. “With this democratization of minimally invasive surgery, patients in rural Nebraska…can get access to some of these procedures without having to travel to Omaha.”
Access to minimally invasive healthcare for all is a concept that Virtual Incision will continue to strive towards. Dixon hopes that this FDA approval will encourage other universities and laboratories to create something even better.
“While this device is really important, this is just the start,” Dixon said. “We create things that have never been seen before, never been done, and it makes an impact.”
“But, it’s also what comes afterward that is impactful.”




Leave a Reply