Summary and The Case
Summary
Background: A 29-year-old man presented to a clinic with infertility and hypogonadism in the setting of morbid obesity. On presentation, he had notable gynecomastia and a low testicular volume. The patient's weight was 154 kg and his height was 168 cm (BMI 54.5 kg/m2). Before referral to the clinic, the patient had been treated with testosterone therapy for 4 months for hypogonadism. This treatment had caused his initially low sperm concentration to fall to undetectable levels.
Investigations: Measurement of reproductive hormone levels, pituitary MRI, and semen analysis.
Diagnosis: Infertility secondary to hypogonadotropic hypogonadism and an elevated estrogen:testosterone ratio.
Management: Treatment with an aromatase inhibitor, anastrozole, led to normalization of the patient's testosterone, luteinizing hormone and follicle-stimulating hormone levels, suppression of serum estradiol levels, and to normalization of spermatogenesis and fertility.
The Case
A 29-year-old man presented to a clinic with a low sperm count in the setting of morbid obesity. The patient and his healthy 32-year-old wife had been unable to conceive after more than 1 year of unprotected intercourse. The patient had been seen by an outside physician and had been diagnosed as having hypogonadism with a low sperm concentration of 2 million/ml (normal concentration ≥20 million/ml). At that time he was started on testosterone therapy, testosterone enantate 200 mg intramuscularly every 2 weeks, for hypogonadism. While the patient was on testosterone therapy, a repeat sperm collection showed a complete absence of sperm (indicating a sperm concentration <2 million/ml). He then presented for a second opinion and further treatment of male infertility. At the time of evaluation at the clinic the patient had ceased testosterone therapy for 2 months.
The patient had gone through normal puberty co-incident with his peers and had never, to his knowledge, fathered a pregnancy. He had no history of testicular trauma, torsion, or infections. The patient denied alcohol, tobacco, marijuana or other illegal drug use, and was taking no prescription medications at the time of referral. He had no history of recent systemic illnesses or considerable weight change. The patient reported being overweight his entire life and denied any episodes of rapid weight gain or loss.
On physical examination, the patient had normal vital signs with a weight of 154 kg, a height of 168 cm, and a BMI of 54.5 kg/m2. Notable findings on examination were the presence of stage IV gynecomastia and morbid central obesity as well as subnormal testicular volumes of 12 cm3 on the right and 8 cm3 on the left. Testicular examination showed no varicocele or hydrocele, and the patient's vas deferens was palpable bilaterally. He had normal male-pattern hair distribution.
Initial laboratory results ( Table 1 ), obtained when the patient presented to the clinic, confirmed the diagnosis of hypogonadism with low total testosterone levels (5.25 nmol/l [151.2 ng/dl]; normal range 7.7-27.3 nmol/l [221.8-786.2 ng/dl]), low levels of calculated free testosterone (102.2 pmol/l [2.9 pg/ml]; normal range 105-490 pmol/l [3.0-14.1 pg/ml]), and normal levels of sex hormone-binding globulin (34.8 nmol/l; normal range 13.0-71.0 nmol/l). Luteinizing hormone (LH) levels were suppressed at <1 IU/l (normal range 1-14 IU/l) and follicle-stimulating hormone (FSH) levels were low at 2 IU/l (normal range 1-14 IU/l). Serum estradiol was within the normal range (73-275 pmol/l [20-75 pg/ml]) with a level of 172 pmol/l (47 pg/ml).
The patient's blood counts and serum chemistry profile, including glucose levels and liver function tests, were within normal limits. In addition, an MRI of the patient's brain was normal and, with the exception of his gonadotropin levels, his pituitary function was also normal ( Table 1 ).
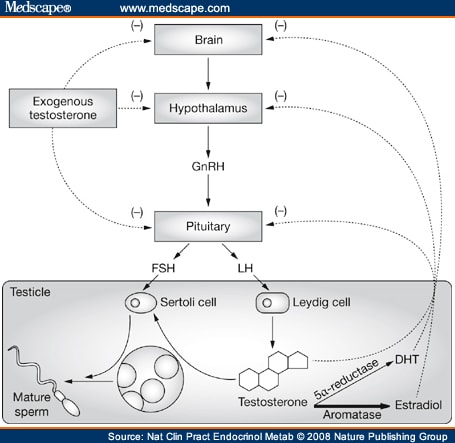
Obesity was felt to be the possible cause of secondary hypogonadism and associated infertility in the patient. He was, therefore, started on treatment with the aromatase inhibitor anastrozole. Aromatase, which converts testosterone to estradiol, is highly expressed in peripheral fat tissue[1] and increased aromatase activity is thought to result in increased estradiol production, which inhibits secretion of LH and FSH from the pituitary. Reduced levels of LH and FSH lead to a reduction in testosterone synthesis and sperm production, and to infertility (Figure 1). When fertility is desired in men with hypogonadism, treatment with testosterone is not appropriate because exogenous testosterone further inhibits gonadotropin secretion and impairs spermatogenesis in men. To counteract the physiologic effects of elevated estradiol in the described patient, treatment with anastrozole was initiated.[2] This treatment normalized his serum testosterone levels and spermatogenesis ( Table 1 ), and after 6 months of therapy his wife became pregnant. At the time of writing, the couple is eagerly awaiting the birth of their first child. Now that the couple has achieved a pregnancy, the therapeutic plan is to discontinue anastrozole as there are limited long-term safety data on the use of this agent in men. The drug could be restarted if another pregnancy is desired in the future. After the cessation of anastrozole therapy a repeat semen analysis is planned in order to verify the treatment effect.
Figure 1.
The hypothalamic-pituitary-gonadal axis in men and the impact of testosterone therapy and estradiol on spermatogenesis. In obese patients, increased aromatase activity is thought to result in increased estradiol production. The increased estradiol level inhibits FSH and LH secretion from the pituitary, which results in reduced FSH and LH stimulation of the Sertoli and Leydig cells in the testes and a reduction in testosterone synthesis and sperm production. Administration of exogenous testosterone effectively raises serum testosterone levels. If fertility is desired administration of testosterone is, however, counterproductive because it provides negative feedback to the pituitary and causes inhibition of LH and FSH secretion. Decreased LH and FSH stimulation of the testes further inhibits spermatogenesis. Abbreviations: DHT, dihydrotestosterone; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
Nat Clin Pract Endocrinol Metab. 2008;4(7):415-419. © 2008
Nature Publishing Group











Comments