Cell division is a precise process, but sometimes it can be impaired, allowing diseases such as cancer to develop. Researchers at Okinawa Institute of Science and Technology (OIST) in collaboration with scientists at the University of California, San Diego, (UCSD) have now discovered a molecular mechanism that prevents multiplication of potentially dangerous cells, by measuring the duration of mitosis. Using techniques including live cell imaging to watch cells over time, the team’s newly reported laboratory research demonstrated that this mitosis duration monitoring mechanism, which they’ve called the mitotic stopwatch, gets stronger with time as cells divide, leading to removal of abnormal cells to protect the organism.
“This work shows that cells carefully monitor the time taken to execute mitosis and use that as a filter to eliminate potentially problematic cells,” said Arshad Desai, PhD, a UCSD faculty member in the department of cell and developmental biology. “If a cell takes longer than normal to complete mitosis, then daughter cells will know that their mother struggled to execute mitosis and they’ll stop dividing as a safety measure.”
The team suggests that their findings have potential clinical applications and could eventually aid in the treatment of certain cancers. Some cancers maintain an active mitotic stopwatch, which makes them sensitive to anti-mitotic drugs that play an important role in cancer treatment by targeting cell division. These drugs are currently in clinical use or development.
“If we could determine the activity of the mitotic stopwatch in individual cancers, we might be able to predict how these cancers respond to treatment with anti-mitotic drugs,” said Prof. Franz Meitinger, PhD, head of the Cell Proliferation and Gene Editing Unit at Okinawa Institute of Science and Technology (OIST). Meitinger, together with Hazrat Belal, PhD a researcher at the unit, are first authors of the team’s published report in Science, titled “Control of cell proliferation by memories of mitosis.” In the paper the team stated “… stopwatch status may influence the efficacy of therapeutic agents currently in use or being developed to target mitotic processes and could serve as a potential biomarker for their use in cancer treatment.”
Every day our cells are hard at work multiplying. Mitosis is one of the most important phases in the cell cycle. During this phase a cell’s DNA is split into two equal sets of chromosomes which are divided correctly into two genetically identical daughter cells.
Normally, when a cell divides, it makes exact copies of the chromosomes so each of the two new daughter cells receives a perfect copy. However, sometimes one cell might get too many chromosomes and the other might not get enough, a phenomenon known as chromosome missegregation.
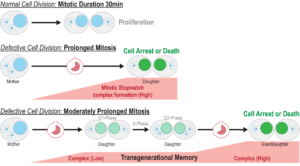
Mitosis usually takes around 30 minutes to complete, but when cells have a defect, they need more time to organize the chromosomes and segregate them to the daughter cells. “Mitosis is a complex event that occurs within a tightly constrained time frame,” the authors wrote. “Prolonged mitosis is a sign of problems that can lead to chromosome missegregation, a trigger event for genomic instability.”
Delays in mitosis trigger what the researchers have called the mitotic stopwatch—a complex that forms when the cells experience unusual, prolonged mitosis. “Because the mechanism monitoring mitotic duration acts in an analog manner and is proportional to time, we refer to it as the mitotic stopwatch,” the authors explained. “This complex doesn’t form during a normal mitosis, only when it takes longer,” Meitinger stated. “The defects in the cells are not directly recognized by the cells, but what the cells can measure is how long they spend in mitosis, and they use this information to understand how well mitosis happens. We wanted to understand how the molecular mechanism protects the organism from cancer development.”
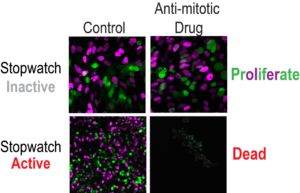
The cells were then observed over the three day period to determine their fate. “After inhibitor washout, cells completed mitosis, and the resulting daughter cells were followed for 48 hours to determine whether they arrested, underwent apoptosis, or continued to proliferate,” the team noted. This method allowed the researchers to show how cells detect prolonged mitosis and subsequently initiate cell arrest or death.
The researchers discovered that the stopwatch is made up of a biochemical pathway that continually surveils the amount of time spent in mitosis. The pathway features a “memory” function that sums up mitosis delays from one generation to the next. They found that mitotic stopwatch complex consists of three proteins, p53 binding protein 1, USP28 and p53 itself. These proteins only interact during an unusually long mitosis, and during this prolonged mitosis, more and more of the complex is formed.
The experiments showed that the pathway works as a quality control mechanism that “remembers” mitotic time. Even cell divisions that are sequentially delayed by as little as 20 minutes are labeled as risky, they found. The complex starts forming 30 minutes after mitosis starts, and after the cell exits extended mitosis it becomes active in the new daughter cells. “We show here that mitotic extension leads to the formation of p53-binding protein 1 (53BP1)–ubiquitin-specific protease 28 (USP28)–p53 protein complexes that are transmitted to, and stably retained by, daughter cells,” they reported. This activation triggers other factors that can either permanently arrest or kill the cells, dependent on cell type. The more complex formed, the stronger the result.
The scientists in addition found that a kinase enzyme called PLK1 is responsible for triggering the formation of the complex. PLK1 is active during normal mitosis, but for unknown reasons only induces complex formation during prolonged mitosis. “This work shows that the degree of mitotic extension is encoded through the PLK1 kinase–dependent assembly of mitotic stopwatch complexes that are transmitted to daughter cells,” they commented.
The researchers believe the crucial 30 minutes of mitotic time could be evolution’s solution to quickly getting through a vital but potentially dangerous part of life when cells are vulnerable. Karen Oegema, PhD, in the USCD School of Biological Sciences and School of Medicine, likens cancers to an alien in our bodies that we are constantly fighting off using surveillance mechanisms like the stopwatch. Importantly, the researchers demonstrated that the stopwatch mechanism is switched “off” by many types of cancers, effectively allowing them to tolerate aberrant genomes that undergo longer and problematic mitoses.
“Our research suggests that measuring mitosis time is a mechanism that was developed as a way to protect us,” said Oegema, also a Cell and Developmental Biology Department professor. “Essentially, it’s another tumor suppression function tied to p53’s job to protect against problematic cells.”
The tumor suppressor protein p53 stops the growth of potentially damaged cells and prevents their proliferation. The reported work showed that when the complex is formed it can stabilize and activate p53, which can then act as a transcription factor—a protein that can act to turn genes off and on, ensuring they are correctly expressed in the right cells at the right time.
The discoveries provide new insights into the roles of these proteins during prolonged mitosis and in the removal of potentially dangerous cells that can cause cancer. “When a little bit of the complex is made which is not enough to arrest the cells, the complex stays stable even in the granddaughter cells and accumulates,” Meitinger stated. “The granddaughter cells can remember the moderately prolonged state of mitosis in the grandmother cells.”
While every cell in our body has the potential for mitotic delay, it is less common in normal cells. A mitotic delay is more likely to happen in damaged cells, and the mitotic stopwatch is likely to act as a surveillance mechanism that removes these cells. In cancer cells there is often an even longer and flawed mitosis. In these cells this pathway is often inactive because it has mutated to have flawed mitosis, driving cancer development.
The scientists used a technique called CRISPR-Cas9 to turn off certain genes and then studied the effects on the p53 protein. They found that some gene mutations can stop the protein from working properly. To understand this better, they are now studying the proteins that form the mitotic stopwatch complex to see how they interact under different conditions.
The authors wrote, “Because stopwatch function relies on p53, the stopwatch is predicted to be inactive in the ~50% of human cancers that harbor p53 mutations. This expectation was confirmed in three p53-mutant cancer-derived cell lines … Compromised stopwatch function is likely important for the tolerance of problematic mitoses that are contributors to and a consequence of the aneuploidy and genomic instability that is characteristic of cancers.”
Belal noted that the most challenging part of the experiment was tracking the cells, as they move a lot and sometimes go out of frame under the microscope. Each experiment required the analysis of at least 160 individual cells, and many experiments had to be conducted to determine the mechanism of the mitotic stopwatch and its functionality across normal and cancer cell types. Every cell had to be analyzed individually. This meticulous process allowed the scientists to fully understand how the cells respond to extended mitosis in different situations.
In conclusion, the team stated, “This work shows that the degree of mitotic extension is encoded through the PLK1 kinase–dependent assembly of mitotic stopwatch complexes that are transmitted to daughter cells. The ability of this mechanism to detect subtle repeated extensions of mitosis functions as a fidelity filter in a proliferative cell population and may explain its frequent inactivation in cancers, as well as the classification of stopwatch complex components as tumor suppressors.”