Targeting Strategies
Two basic requirements should be realized in the design of nanocarriers to achieve effective drug delivery (Figure 2). First, drugs should be able to reach the desired tumor sites after administration with minimal loss to their volume and activity in blood circulation. Second, drugs should only kill tumor cells without harmful effects to healthy tissue.[146] These requirements may be enabled using two strategies: passive and active targeting of drugs.[147]
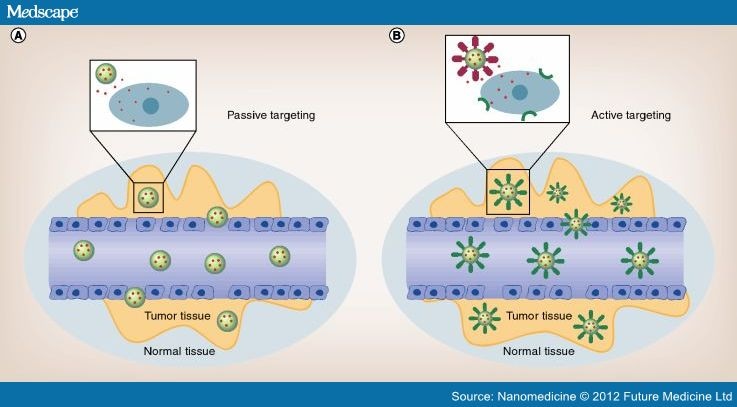
Figure 2.
Passive and active targeting.
By the enhanced permeability and retention effect, nanoparticles (NPs) can be passively extravasated through leaky vascularization, allowing their accumulation at the tumor region (A). In this case, drugs may be released in the extracellular matrix and then diffuse through the tissue. Active targeting (B) can enhance the therapeutic efficacy of drugs by the increased accumulation and cellular uptake of NPs through receptor-mediated endocytosis. NPs can be engineered to incorporate ligands that bind to endothelial cell surface receptors. In this case, the enhanced permeability and retention effect does not pertain, and the presence of leaky vasculature is not required.
Passive Targeting
Passive targeting takes advantage of the unique pathophysiological characteristics of tumor vessels, enabling nanodrugs to accumulate in tumor tissues. Typically, tumor vessels are highly disorganized and dilated with a high number of pores, resulting in enlarged gap junctions between endothelial cells and compromised lymphatic drainage. The 'leaky' vascularization, which refers to the EPR effect, allows migration of macromolecules up to 400 nm in diameter into the surrounding tumor region.[147–149] One of the earliest nanoscale technologies for passive targeting of drugs was based on the use of liposomes. More advanced liposomes are coated with a synthetic polymer that protects the agents from immune destruction.[150]
Moreover, the EPR effect, the microenvironment surrounding tumor tissue, is different from that of healthy cells, a physiological phenomenon that also supports passive targeting. Based on the high metabolic rate of fast-growing tumor cells, they require more oxygen and nutrients. Consequently, glycolysis is stimulated to obtain extra energy, resulting in an acidic environment.[151] Taking advantage of this, pH-sensitive liposomes have been designed to be stable at physiological pH 7.4, but degraded to release drug molecules at the acidic pH.[152]
Although passive targeting approaches form the basis of clinical therapy, they suffer from several limitations. Ubiquitously targeting cells within a tumor is not always feasible because some drugs cannot diffuse efficiently, and the random nature of the approach makes it difficult to control the process. The passive strategy is further limited because certain tumors do not exhibit an EPR effect, and the permeability of vessels may not be the same throughout a single tumor.[153]
Active Targeting
One way to overcome the limitations of passive targeting is to attach affinity ligands (antibodies,[154] peptides,[155] aptamers[156] or small molecules[157] that only bind to specific receptors on the cell surface) to the surface of the nanocarriers by a variety of conjugation chemistries. Nanocarriers will recognize and bind to target cells through ligand–receptor interactions by the expression of receptors or epitopes on the cell surface. In order to achieve high specificity, those receptors should be highly expressed on tumor cells, but not on normal cells. Furthermore, the receptors should homogeneously express and should not be shed into the blood circulation. Internalization of targeting conjugates can also occur by receptor-mediated endocytosis after binding to target cells, facilitating drug release inside the cells. Based on the receptor-mediated endocytosis mechanism, targeting conjugates bind with their receptors first, followed by plasma membrane enclosure around the ligand–receptor complex to form an endosome. The newly formed endosome is transferred to specific organelles, and drugs could be released by acidic pH or enzymes. Although the active targeting strategy looks intriguing, nanodrugs currently approved for clinical use are relatively simple and generally lack active targeting or triggered drug release components. Moreover, nanodrugs currently under clinical development lack specific targeting. To fully explore the application of targeted drug delivery, we need to investigate whether the specific diseases are the correct application for targeting, whether the properties of the therapeutic drugs, as well as their site and mode of action, are suited for targeting and whether the delivery vehicles are optimal for product development.[158]
Nanomedicine. 2012;7(8):1253-1271. © 2012 Future Medicine Ltd.