Scientists at ETH Zurich report that they have engineered bacteria in the laboratory to efficiently use methanol. The metabolism of these bacteria can now be tapped into to produce valuable products currently made by the chemical industry from fossil fuels, according to the researchers, whose research paper “A synthetic methylotrophic Escherichia coli as a chassis for bioproduction from methanol” appears in Nature Catalysis.
“Methanol synthesized from captured greenhouse gases is an emerging renewable feedstock with great potential for bioproduction. Recent research has raised the prospect of methanol bioconversion to value-added products using synthetic methylotrophic Escherichia coli, as its metabolism can be rewired to enable growth solely on the reduced one-carbon compound,” write the investigators.
“Here we describe the generation of an E. coli strain that grows on methanol at a doubling time of 4.3 h—comparable to many natural methylotrophs. To establish bioproduction from methanol using this synthetic chassis, we demonstrate biosynthesis from four metabolic nodes from which numerous bioproducts can be derived: lactic acid from pyruvate, polyhydroxybutyrate from acetyl coenzyme A, itaconic acid from the tricarboxylic acid cycle and p-aminobenzoic acid from the chorismate pathway.
“In a step towards carbon-negative chemicals and valorizing greenhouse gases, our work brings synthetic methylotrophy in E. coli within reach of industrial applications.”
To produce various chemicals such as plastics, dyes or artificial flavors, the chemical industry currently relies heavily on fossil resources such as crude oil.
“Globally, it consumes 500 million tons per year, or more than one million tons per day,” notes Julia Vorholt-Zambelli, PhD, professor at the Institute of Microbiology at ETH Zurich, whose team is looking for ways to reduce the chemical industry’s dependence on fossil fuels. “Since these chemical conversions are energy-intensive, the true CO2 footprint of the chemical industry is even six to ten times larger, amounting to about five percent of total emissions globally.”
Green methanol
Bacteria that feed on methanol (methylotrophs) are at the center of these efforts. Containing just a single carbon atom, methanol is one of the simplest organic molecules and can be synthesized from the greenhouse gas carbon dioxide and water. If the energy for this synthesis reaction comes from renewable sources, the methanol is termed “green.”
“There are natural methylotrophs but using them industrially remains difficult despite considerable research effort,” says Michael Reiter, PhD, a postdoctoral researcher in Vorholt-Zambelli’s research group, which instead works with the biotechnologically well-understood model bacterium Escherichia coli. Vorholt-Zambelli’s team has been pursuing the idea of equipping the model bacterium, which grows on sugar, with the ability to metabolize methanol for several years.
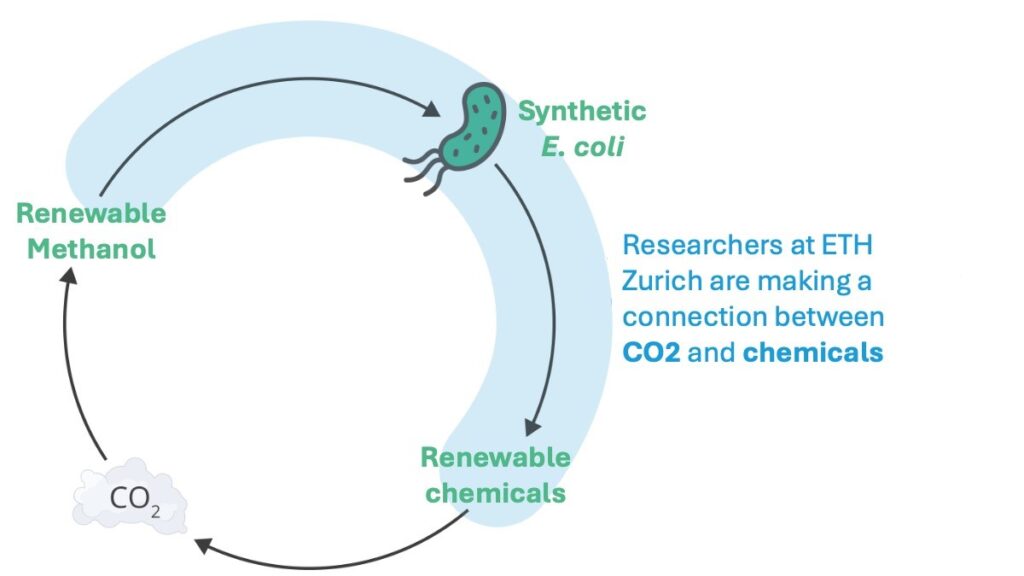
They continued to grow the bacteria under special conditions in the laboratory for more than a year until the microbes could produce all cell components from methanol. Over the course of around 1,000 more generations, these synthetic methylotrophs became increasingly efficient, eventually doubling every four hours when fed only with methanol. “The improved growth rate makes the bacteria economically interesting,” explains Vorholt-Zambelli.
Optimization through loss of function
Several randomly occurring mutations are responsible for the increased efficiency of methanol utilization. Most of these mutations resulted in the loss of function of various genes. This is surprising at first glance, but upon closer inspection, it becomes apparent that the cells can save energy thanks to the loss of function of the genes. For example, some mutations cause the reverse reactions of important biochemical reactions to fail. “This abolishes superfluous chemical conversions and optimizes the metabolic flux in the cells,” the researchers write.
To explore the potential of synthetic methylotrophs for the biotechnological production of industrially relevant bulk chemicals, Vorholt-Zambelli and her team have equipped the bacteria with additional genes for four different biosynthetic pathways. In their study, they now show that the bacteria indeed produced the desired compounds in all cases.
For the researchers, this is clear evidence that their engineered bacteria can deliver on what was originally promised: the microbes are a kind of highly versatile production platform into which biosynthesis modules can be inserted according to the “plug-and-play” principle, prompting the bacteria to convert methanol into desired biochemical substances.
However, the researchers still need to significantly increase the yield and productivity to enable economically viable use of the bacteria. Vorholt-Zambelli and her team recently received an innovation fund “to further expand plans towards applications and to select products to focus on first,” says Vorholt-Zambelli.


