A team of scientists led by the Icahn School of Medicine at Mount Sinai has successfully reversed congenital blindness in mice by changing supportive cells in the retina called Müller glia into rod photoreceptors. The findings, published in the journal Nature, advance efforts toward regenerative therapies for blinding diseases such as age-related macular degeneration and retinitis pigmentosa.
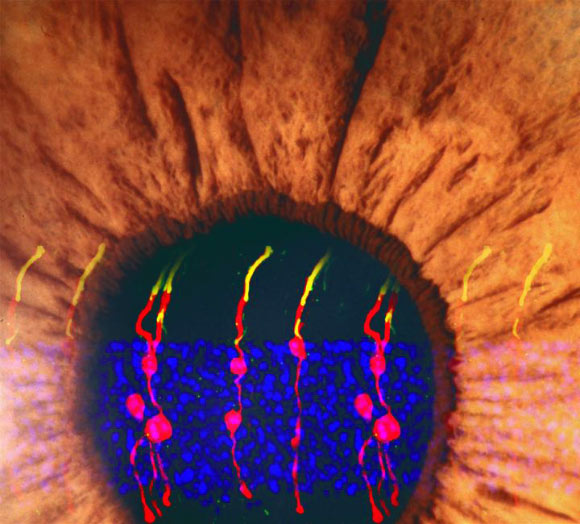
An artist’s rendering incorporates the images of the Müller glia-derived rod photoreceptors. These photoreceptors were structurally no different from real photoreceptors and they became integrated within the circuitry of the visual pathway, from the retina to the brain. Image credit: Bo Chen.
Photoreceptors are light-sensitive cells in the retina in the back of the eye that signal the brain when activated.
In mammals, including mice and humans, photoreceptors fail to regenerate on their own. Like most neurons, once mature they don’t divide.
Researchers have long studied the regenerative potential of Müller glia cells (MGCs) because in some species, such as zebrafish, they divide in response to injury and can turn into photoreceptors and other retinal neurons. The zebrafish can thus regain vision after severe retinal injury.
In mammals, however, MGCs do not spontaneously re-enter the cell cycle to generate a population of stem or progenitor cells that differentiate into retinal neurons.
As a result, in patients with diseases like macular degeneration or retinitis pigmentosa that kill retinal cells, the disease progression is often irreversible.
Dr. Bo Chen from the Icahn School of Medicine at Mount Sinai and colleagues hypothesized if they could somehow reactivate MGCs and bring back vision.
“From a practical standpoint, if you’re trying to regenerate the retina to restore a person’s vision, it is counterproductive to injure it first to activate MGCs,” Dr. Chen said.
“We wanted to see if we could program MGCs to become rod photoreceptors in a living mouse without having to injure its retina.”
The researchers did a two-step gene transfer to reprogram MGCs into blind mice.
First, they activated dormant stem cells to become active stem cells. The second step involved another gene transfer to help stem cells develop into rod photoreceptor cells.
After this two-step reprogramming, the team found that new rod photoreceptors were generated and integrated into the existing retinal structure, instead of floating around and being ineffective.
The study authors saw no difference between these new cells and real rod photoreceptor cells.
These cells sensed light, which then triggered information to be sent to the visual cortex (brain) and restored function of the visual pathway.
Between four and six weeks after the reprogramming, the blind mice were able to sense light and regained their vision.
While vision was restored to some degree, the researchers could not measure the degree of improvement, and must do further testing to find this out.
“This study opens a new pathway for potentially treating blinding diseases by manipulating our own regenerative capability to self-repair,” Dr. Chen said.
“This is the first step to finding promising cures for patients that involve self-repair as opposed to medicine or invasive procedures.”
“This is the first report of scientists reprogramming MGCs to become functional rod photoreceptors in the mammalian retina,” said Dr. Thomas Greenwell, program director for retinal neuroscience at the National Eye Institute.
“Rods allow us to see in low light, but they may also help preserve cone photoreceptors, which are important for color vision and high visual acuity. Cones tend to die in later-stage eye diseases. If rods can be regenerated from inside the eye, this might be a strategy for treating diseases of the eye that affect photoreceptors.”
_____
Kai Yao et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature, published online August 15, 2018; doi: 10.1038/s41586-018-0425-3







