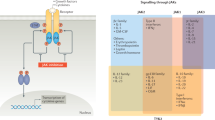
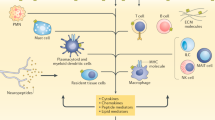
Abstract
Despite the introduction of numerous biologic agents for the treatment of rheumatoid arthritis (RA) and other forms of inflammatory arthritis, low-dose methotrexate therapy remains the gold standard in RA therapy. Methotrexate is generally the first-line drug for the treatment of RA, psoriatic arthritis and other forms of inflammatory arthritis, and it enhances the effect of most biologic agents in RA. Understanding the mechanism of action of methotrexate could be instructive in the appropriate use of the drug and in the design of new regimens for the treatment of RA. Although methotrexate is one of the first examples of intelligent drug design, multiple mechanisms potentially contribute to the anti-inflammatory actions of methotrexate, including the inhibition of purine and pyrimidine synthesis, transmethylation reactions, translocation of nuclear factor-κB (NF-κB) to the nucleus, signalling via the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway and nitric oxide production, as well as the promotion of adenosine release and expression of certain long non-coding RNAs.
Key points
-
Methotrexate polyglutamates inhibit aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase (ATIC), leading to intracellular accumulation of AICAR and increased adenosine release; adenosine binds to cell surface receptors and suppresses many inflammatory and immune reactions.
-
Methotrexate inhibits dihydrofolate reductase, preventing the reduction of dihydrobiopterin (BH2) to tetrahydrobiopterin (BH4), leading to nitric oxide synthase uncoupling and increased sensitivity of T cells to apoptosis, thereby diminishing immune responses.
-
Methotrexate inhibits activation of nuclear factor-κB (NF-κB) by increasing both adenosine release and activation of adenosine receptor A2a and by inhibiting the reduction of BH2 to BH4.
-
Methotrexate increases the expression of long intergenic non-coding RNA p21 (lincRNA-p21), which is a multifunction long non-coding RNA that regulates, both directly and indirectly, a variety of critical immune and inflammatory processes.
-
By modulating cell-specific signalling pathways, methotrexate inhibits important pro-inflammatory properties of major cell lineages involved in rheumatoid arthritis pathogenesis, including T cells, macrophages, endothelial cells and fibroblast-like synoviocytes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weinblatt, M. E. Methotrexate: who would have predicted its importance in rheumatoid arthritis? Arthritis Res. Ther. 20, 103 (2018).
Singh, J. A. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 68, 1–25 (2016).
Aaltonen, K. J. et al. Do biologic drugs affect the need for and outcome of joint replacements in patients with rheumatoid arthritis? A register-based study. Semin. Arthritis Rheum. 43, 55–62 (2013).
Asai, S. et al. Effects of concomitant methotrexate on large joint replacement in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a multicenter retrospective cohort study in Japan. Arthritis Care Res. 67, 1363–1370 (2015).
Asai, S. et al. Concomitant methotrexate protects against total knee arthroplasty in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors. J. Rheumatol. 42, 2255–2260 (2015).
Herman, R. A., Veng-Pedersen, P., Hoffman, J., Koehnke, R. & Furst, D. E. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J. Pharm. Sci. 78, 165–171 (1989).
Schiff, M. H. & Sadowski, P. Oral to subcutaneous methotrexate dose-conversion strategy in the treatment of rheumatoid arthritis. Rheumatol. Int. 37, 213–218 (2017).
Kremer, J. M., Galivan, J., Streckfuss, A. & Kamen, B. Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis Rheum. 29, 832–835 (1986).
Chabner, B. A. et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J. Clin. Invest. 76, 907–912 (1985).
Steffen, J. A. & Stolzmann, W. M. Studies on in vitro lymphocyte proliferation in cultures synchronized by the inhibition of DNA synthesis. I. Variability of S plus G2 periods of first generation cells. Exp. Cell Res. 56, 453–460 (1969).
Morgan, S. L., Baggott, J. E., Lee, J. Y. & Alarcon, G. S. Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: implications for cardiovascular disease prevention. J. Rheumatol. 25, 441–446 (1998).
Morgan, S. L. et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann. Int. Med. 121, 833–841 (1994).
Morgan, S. L. et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 33, 9–18 (1990).
Shiroky, J. B. et al. Low-dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis. Results of a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 36, 795–803 (1993).
Shea, B. Folic acid or folinic acid for reducing side effects of methotrexate for people with rheumatoid arthritis. J. Evid. Based Med. 6, 202–203 (2013).
Joyce, D. A., Will, R. K., Hoffman, D. M., Laing, B. & Blackbourn, S. J. Exacerbation of rheumatoid arthritis in patients treated with methotrexate after administration of folinic acid. Ann. Rheum. Dis. 50, 913–914 (1991).
Tishler, M., Caspi, D., Fishel, B. & Yaron, M. The effects of leucovorin (folinic acid) on methotrexate therapy in rheumatoid arthritis patients. Arthritis Rheum. 31, 906–908 (1988).
Yukioka, K. et al. Polyamine levels in synovial tissues and synovial fluids of patients with rheumatoid arthritis. J. Rheumatol. 19, 689–692 (1992).
Nesher, G. & Moore, T. L. The in vitro effects of methotrexate on peripheral blood mononuclear cells. Modulation by methyl donors and spermidine. Arthritis Rheum. 33, 954–959 (1990).
Furumitsu, Y. et al. Levels of urinary polyamines in patients with rheumatoid arthritis. J. Rheumatol. 20, 1661–1665 (1993).
Nesher, G., Osborn, T. G. & Moore, T. L. In vitro effects of methotrexate on polyamine levels in lymphocytes from rheumatoid arthritis patients. Clin. Exp. Rheumatol. 14, 395–399 (1996).
Nesher, G., Moore, T. L. & Dorner, R. W. In vitro effects of methotrexate on peripheral blood monocytes: modulation by folinic acid and S-adenosylmethionine. Ann. Rheum. Dis. 50, 637–641 (1991).
Chan, E. S. & Cronstein, B. N. Methotrexate–how does it really work? Nat. Rev. Rheumatol. 6, 175–178 (2010).
Allegra, C. J., Drake, J. C., Jolivet, J. & Chabner, B. A. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc. Natl Acad. Sci. USA 82, 4881–4885 (1985).
Cronstein, B. N., Naime, D. & Ostad, E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 92, 2675–2682 (1993).
Cronstein, B. N. & Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 13, 41–51 (2017).
Montesinos, M. C. et al. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 43, 656–663 (2000).
Montesinos, M. C. et al. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5’-nucleotidase: findings in a study of ecto-5’-nucleotidase gene-deficient mice. Arthritis Rheum. 56, 1440–1445 (2007).
Montesinos, M. C., Desai, A. & Cronstein, B. N. Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res. Ther. 8, R53 (2006).
Montesinos, M. C. et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 48, 240–247 (2003).
Riksen, N. P. et al. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann. Rheum. Dis. 65, 465–470 (2006).
Nesher, G., Mates, M. & Zevin, S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis Rheum. 48, 571–572 (2003).
Benito-Garcia, E. et al. Dietary caffeine intake does not affect methotrexate efficacy in patients with rheumatoid arthritis. J. Rheumatol. 33, 1275–1281 (2006).
Allard, D., Turcotte, M. & Stagg, J. Targeting A2 adenosine receptors in cancer. Immunol. Cell Biol. 95, 333–339 (2017).
Peres, R. S. et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 112, 2509–2514 (2015).
Bitoun, S. et al. Methotrexate and BAFF interaction prevents immunization against TNF inhibitors. Ann. Rheum. Dis. 77, 1463–1470 (2018).
Chalupsky, K. & Cai, H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 102, 9056–9061 (2005).
Crabtree, M. J., Tatham, A. L., Hale, A. B., Alp, N. J. & Channon, K. M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling – relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 284, 28128–28136 (2009).
Sugiyama, T., Levy, B. D. & Michel, T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J. Biol. Chem. 284, 12691–12700 (2009).
Spurlock, C. F. et al. Increased sensitivity to apoptosis induced by methotrexate is mediated by JNK. Arthritis Rheum. 63, 2606–2616 (2011).
Spurlock, C. F., Tossberg, J. T., Fuchs, H. A., Olsen, N. J. & Aune, T. M. Methotrexate increases expression of cell cycle checkpoint genes via JNK activation. Arthritis Rheum. 64, 1780–1789 (2012).
Spurlock, C. F. et al. Methotrexate-mediated inhibition of nuclear factor κB activation by distinct pathways in T cells and fibroblast-like synoviocytes. Rheumatology 54, 178–187 (2015).
Rinn, J. L. & Chang, H. Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81, 145–166 (2012).
Huarte, M. et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419 (2010).
Weinblatt, M. E. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans. Am. Clin. Climatol. Assoc. 124, 16–25 (2013).
Yang, F., Zhang, H. F., Mei, Y. D. & Wu, M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol. Cell 53, 88–100 (2014).
Yoon, J. H. et al. LincRNA-p21 suppresses target mRNA translation. Mol. Cell 47, 648–655 (2012).
Spurlock, C. F., Tossberg, J. T., Matlock, B. K., Olsen, N. J. & Aune, T. M. Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol. 66, 2947–2957 (2014).
Malemud, C. J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 10, 117–127 (2018).
Gremese, E., Alivernini, S., Tolusso, B., Zeidler, M. P. & Ferraccioli, G. JAK inhibition by methotrexate (and csDMARDs) may explain clinical efficacy as monotherapy and combination therapy. J. Leukoc. Biol. 106, 1063–1068 (2019).
Thomas, S. et al. Methotrexate Is a JAK/STAT pathway inhibitor. PLoS One 10, e0130078 (2015).
Hassanian, S. M., Dinarvand, P. & Rezaie, A. R. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. J. Cell Physiol. 229, 1292–1300 (2014).
Yang, J. et al. Adenosine increases LPS-induced nuclear factor kappa B activation in smooth muscle cells via an intracellular mechanism and modulates it via actions on adenosine receptors. Acta Physiol. 210, 590–599 (2014).
Mediero, A., Perez-Aso, M. & Cronstein, B. N. Activation of adenosine A2A receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br. J. Pharmacol. 169, 1372–1388 (2013).
Tang, L. M. et al. Activation of adenosine A2A receptor attenuates inflammatory response in a rat model of small-for-size liver transplantation. Transpl. Proc. 42, 1915–1920 (2010).
Di Paola, R. et al. Adenosine A2A receptor-selective stimulation reduces signaling pathways involved in the development of intestine ischemia and reperfusion injury. Shock 33, 541–551 (2010).
Ramanathan, M., Pinhal-Enfield, G., Hao, I. & Leibovich, S. J. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol. Biol. Cell 18, 14–23 (2007).
Zernecke, A. et al. CD73/ecto-5’-nucleotidase protects against vascular inflammation and neointima formation. Circulation 113, 2120–2127 (2006).
Sands, W. A., Martin, A. F., Strong, E. W. & Palmer, T. M. Specific inhibition of nuclear factor-κB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol. Pharmacol. 66, 1147–1159 (2004).
Lukashev, D., Ohta, A., Apasov, S., Chen, J. F. & Sitkovsky, M. Cutting edge: physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J. Immunol. 173, 21–24 (2004).
Bshesh, K. et al. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J. Leukoc. Biol. 72, 1027–1036 (2002).
Plant, D. et al. A genetic marker at the OLIG3/TNFAIP3 locus associates with methotrexate continuation in early inflammatory polyarthritis: results from the Norfolk arthritis register. Pharmacogenomics J. 12, 128–133 (2012).
Municio, C. et al. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann. Rheum. Dis. 75, 2157–2165 (2016).
Municio, C. et al. Methotrexate limits inflammation through an A20-dependent cross-tolerance mechanism. Ann. Rheum. Dis. 77, 752–759 (2018).
Spurlock, C. F. III et al. Methotrexate-mediated inhibition of nuclear factor κB activation by distinct pathways in T cells and fibroblast-like synoviocytes. Rheumatology 54, 178–187 (2015).
Vousden, K. H. & Prives, C. Blinded by the light: the growing complexity of p53. Cell 137, 413–431 (2009).
Kang, C. et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 (2015).
Cooks, T. et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23, 634–646 (2013).
Menendez, D., Shatz, M. & Resnick, M. A. Interactions between the tumor suppressor p53 and immune responses. Curr. Opin. Oncol. 25, 85–92 (2013).
Cooks, T., Harris, C. C. & Oren, M. Caught in the cross fire: p53 in inflammation. Carcinogenesis 35, 1680–1690 (2014).
Takatori, H., Kawashima, H., Suzuki, K. & Nakajima, H. Role of p53 in systemic autoimmune diseases. Crit. Rev. Immunol. 34, 509–516 (2014).
Rackov, G. et al. p21 mediates macrophage reprogramming through regulation of p50-p50 NF-κB and IFN-β. J. Clin. Invest. 126, 3089–3103 (2016).
Wu, Z. H., Shi, Y. L., Tibbetts, R. S. & Miyamoto, S. Molecular linkage between the kinase ATM and NF-κB signaling in response to genotoxic stimuli. Science 311, 1141–1146 (2006).
Zhang, T. et al. p53 predominantly regulates IL-6 production and suppresses synovial inflammation in fibroblast-like synoviocytes and adjuvant-induced arthritis. Arthritis Res. Ther. 18, 271 (2016).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Zheng, S. J., Lamhamedi-Cherradi, S. E., Wang, P., Xu, L. Y. & Chen, Y. H. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 54, 1423–1428 (2005).
Okuda, Y., Okuda, M. & Bernard, C. C. A. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 135, 29–37 (2003).
Kawashima, H. et al. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J. Immunol. 191, 3614–3623 (2013).
Munoz-Fontela, C., Mandinova, A., Aaronson, S. A. & Lee, S. W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 16, 741–750 (2016).
Bartok, B. & Firestein, G. S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010).
Olsen, N. J., Spurlock, C. F. & Aune, T. M. Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis Res. Ther. 16, R17 (2014).
Merrill, J. T. et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 40, 1308–1315 (1997).
Chagoya de Sanchez, V. et al. Day-night variations of adenosine and its metabolizing enzymes in the brain cortex of the rat–possible physiological significance for the energetic homeostasis and the sleep-wake cycle. Brain Res. 612, 115–121 (1993).
Chagoya de Sanchez, V. Circadian variations of adenosine and of its metabolism. Could adenosine be a molecular oscillator for circadian rhythms? Can. J. Physiol. Pharmacol. 73, 339–355 (1995).
Chagoya de Sanchez, V. et al. Temporal variations of adenosine metabolism in human blood. Chronobiol. Int. 13, 163–177 (1996).
Chan, E. S. et al. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 148, 1144–1155 (2006).
Che, J., Chan, E. S. & Cronstein, B. N. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol. Pharmacol. 72, 1626–1636 (2007).
Morgan, S. L., Baggott, J. E., Koopman, W. J., Krumdieck, C. L. & Alarcon, G. S. Folate supplementation and methotrexate. Ann. Rheum. Dis. 52, 315–316 (1993).
Alarcon, G. S. & Morgan, S. L. Guidelines for folate supplementation in rheumatoid arthritis patients treated with methotrexate: comment on the guidelines for monitoring drug therapy. Arthritis Rheum. 40, 391 (1997).
Roszkiewicz, J. & Smolewska, E. In the pursuit of methotrexate treatment response biomarker in Juvenile idiopathic arthritis — are we getting closer to personalised medicine? Curr. Rheumatol. Rep. 19, 19 (2017).
Taylor, J. C. et al. Genome-wide association study of response to methotrexate in early rheumatoid arthritis patients. Pharmacogenomics J. 18, 528–538 (2018).
Plant, D. et al. Profiling of gene expression biomarkers as a classifier of methotrexate nonresponse in patients with rheumatoid arthritis. Arthritis Rheumatol. 71, 678–684 (2019).
de Rotte, M. et al. Development and validation of a prognostic multivariable model to predict insufficient clinical response to methotrexate in rheumatoid arthritis. PLoS One 13, e0208534 (2018).
Sergeant, J. C. et al. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res. Ther. 20, 147 (2018).
Teitsma, X. M. et al. Inadequate response to treat-to-target methotrexate therapy in patients with new-onset rheumatoid arthritis: development and validation of clinical predictors. Ann. Rheum. Dis. 77, 1261–1267 (2018).
Szodoray, P. et al. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun. Rev. 9, 140–143 (2010).
Luban, S. & Li, Z. G. Citrullinated peptide and its relevance to rheumatoid arthritis: an update. Int. J. Rheum. Dis. 13, 284–287 (2010).
Gallucci, S. & Matzinger, P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13, 114–119 (2001).
Matzinger, P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 8, 11–13 (2007).
Gubner, R., August, S. & Ginsberg, V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am. J. Med. Sci. 221, 176–182 (1951).
Weinblatt, M. E. et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N. Engl. J. Med. 312, 818–822 (1985).
Williams, H. J. et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis: a controlled clinical trial. Arthritis Rheum. 28, 721–730 (1985).
Kremer, J. M. & Lee, J. K. The safety and efficacy of the use of methotrexate in long-term therapy for rheumatoid arthritis. Arthritis Rheum. 29, 822–831 (1986).
US Food and Drug Administration. Drug approval package. accessdata.fda.gov https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204824Orig2s000TOC.cfm (2014).
US Food and Drug Administration. Rasuvo (methotrexate) injection accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205776Orig1s000TOC.cfm (2015).
Acknowledgements
The work of T.M.A. was supported by grants from the US National Institutes of Health (R21AR063846, R42AI53948, R01AI044924), the American College of Rheumatology ‘Within Our Reach’ grant programme (ACR124405), the US National Center for Advancing Translation Sciences (UL1TR000445) and the US National Science Foundation Graduate Research Fellowship Program.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
B.N.C. declares that he holds equity in Regenosine, a biotech start-up developing therapies for osteoarthritis, and CanFite Biopharma, that he has received grant support from AstraZeneca and Kairos Inc., and that he has a number of patents involving adenosine receptors and hepatic fibrosis, wound healing, bone regeneration and osteoarthritis. T.M.A. declares that he has no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks W. Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Long non-coding RNAs
-
(lncRNAs). A newly discovered class of RNAs that are defined as RNAs of more than 200 base pairs in length, are transcribed from genes but not translated into proteins due to the presence of multiple translational stop codons and, as RNAs, regulate the expression of target genes and proteins to carry out a vast array of biologic functions.
- Warburg effect
-
A shift from oxidative phosphorylation to glycolysis during cell activation.
Rights and permissions
About this article
Cite this article
Cronstein, B.N., Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 16, 145–154 (2020). https://doi.org/10.1038/s41584-020-0373-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-0373-9
This article is cited by
-
Methotrexate treatment strategies for rheumatoid arthritis: a scoping review on doses and administration routes
BMC Rheumatology (2024)
-
Synovium microenvironment-responsive injectable hydrogel inducing modulation of macrophages and elimination of synovial fibroblasts for enhanced treatment of rheumatoid arthritis
Journal of Nanobiotechnology (2024)
-
Discovering therapeutic possibilities for polycystic ovary syndrome by targeting XIST and its associated ceRNA network through the analysis of transcriptome data
Scientific Reports (2024)
-
Overexpression of Synoviolin and miR-125a-5p, miR-19b-3p in peripheral blood of rheumatoid arthritis patients after treatment with conventional DMARDs and methylprednisolone
Clinical Rheumatology (2024)
-
Seven-chain adaptive immune receptor repertoire analysis in rheumatoid arthritis reveals novel features associated with disease and clinically relevant phenotypes
Genome Biology (2024)