Abstract
Introduction
Germline variants in androgen metabolism genes may influence clinical response to androgen deprivation therapy (ADT) in advanced prostate cancer. We sought to investigate the prognostic significance of germline variants in androgen metabolism genes with respect to overall survival (OS) after ADT, and to associate germline variants with tumor genomic features.
Methods
Germline and somatic whole-genome sequencing (WGS) data were evaluated in a cohort of 101 men with metastatic castration-resistant prostate cancer (mCRPC). Survival analyses were performed to identify polymorphisms associated with impaired OS after primary ADT. Germline variants found to be prognostic of OS were associated with tumor somatic DNA-sequence alterations based on WGS performed on paired metastasis biopsies from the same 101 patients. Gene set enrichment analysis was performed based on tumor RNA-sequencing data to identify genomic pathways differentially expressed in patients with germline variants.
Results
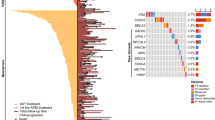
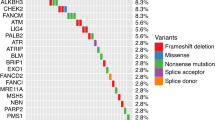
A comprehensive literature review identified 17 candidate polymorphisms in nine androgen metabolism genes that have been previously shown to have an association with response to ADT in prostate cancer. Of these, the variant rs1856888 allele located 13 kb upstream of HSD3B1 was found to be significantly associated with impaired OS (P = 0.029). Variant rs1856888 was commonly co-inherited with the well-characterized HSD3B1(1245A>C) polymorphism, and there was a trend toward shorter median OS in patients with HSD3B1(1245A>C) compared with homozygous wild-type patients (P = 0.052). While HSD3B1 germline variants were not associated with common somatic tumor DNA alterations, they were associated with increased tumor expression of cell proliferation and cell cycle genes.
Conclusions
This study presents a comprehensive assessment of germline variants in androgen metabolism genes and highlights HSD3B1 polymorphisms as prognostic of OS after ADT and associated with an aggressive gene expression tumor profile in mCRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Eeles RA. Genetic predisposition to prostate cancer. Prostate Cancer Prostatic Dis. 1999;2:9–15.
Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomark Prev. 2014;23:2303–10.
Dias A, Kote-Jarai Z, Mikropoulos C, Eeles R. Prostate cancer germline variations and implications for screening and treatment. Cold Spring Harb Perspect Med. 2018;8:a030379.
Fujimoto N, Shiota M, Tomisaki I, Minato A. Gene polymorphism-related individual and interracial differences in the outcomes of androgen deprivation therapy for prostate cancer. Clin Genitourin Cancer. 2017;15:337–42.
Chang K-H, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108:13728–33.
Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Dev Ther. 2012;6:13–18.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Alyamani M, Emamekhoo H, Park S, Taylor J, Almassi N, Upadhyay S, et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Investig. 2018;128:3333–40.
Ross RW, Oh WK, Xie W, Pomerantz M, Nakabayashi M, Sartor O, et al. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2008;26:842–7.
Shiota M, Narita S, Akamatsu S, Fujimoto N, Sumiyoshi T, Fujiwara M, et al. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw Open. 2019;2:e190115.
Almassi N, Reichard C, Li J, Russell C, Perry J, Ryan CJ, et al. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 2018;4:554–7.
Hearn JWD, AbuAli G, Reichard CA, Reddy CA, Magi-Galluzzi C, Chang K-H, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17:1435–44.
Hur J, Schuyler AD, States DJ, Feldman EL. SciMiner: web-based literature mining tool for target identification and functional enrichment analysis. Bioinformatics. 2009;25:838–40.
Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174:758–.e9.
Chen, W. S., Aggarwal, R., Zhang, L., Zhao, S. G., Thomas, G. V., Beer, T. M., et al. Genomic drivers of poor prognosis and enzalutamide resistance in metastatic castration-resistant prostate cancer. Eur Urol. S0302283819302064 2019. https://doi.org/10.1016/j.eururo.2019.03.020.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Lévesque É, Huang S-P, Audet-Walsh É, Lacombe L, Bao B-Y, Fradet Y, et al. Molecular markers in key steroidogenic pathways, circulating steroid levels, and prostate cancer progression. Clin Cancer Res. 2013;19:699–709.
Shiota M, Fujimoto N, Yokomizo A, Takeuchi A, Itsumi M, Inokuchi J, et al. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur J Cancer. 2015;51:1962–9.
Shiota M, Fujimoto N, Yokomizo A, Takeuchi A, Kashiwagi E, Dejima T, et al. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 2016;19:191–6.
Fujimoto N, Kubo T, Inatomi H, Bui HTT, Shiota M, Sho T, et al. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013;16:336–40.
Wang X, Harshman LC, Xie W, Nakabayashi M, Qu F, Pomerantz MM, et al. Association of SLCO2B1 genotypes with time to progression and overall survival in patients receiving androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2016;34:352–9.
Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2011;29:2565–73.
Sharifi N, Hamada A, Sissung T, Danesi R, Venzon D, Baum C, et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102:617–21.
Kanda S, Tsuchiya N, Narita S, Inoue T, Huang M, Chiba S, et al. Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer. 2015;136:74–82.
Shiota M, Fujimoto N, Tsukahara S, Ushijima M, Takeuchi A, Kashiwagi E, et al. The impact of genetic polymorphism on CYP19A1 in androgen-deprivation therapy among Japanese men. Cancer Chemother Pharm. 2019;83:933–8.
Yamada T, Nakayama M, Shimizu T, Nonen S, Nakai Y, Nishimura K, et al. Genetic polymorphisms of CYP17A1 in steroidogenesis pathway are associated with risk of progression to castration-resistant prostate cancer in Japanese men receiving androgen deprivation therapy. Int J Clin Oncol. 2013;18:711–7.
Yu C-C, Huang S-P, Lee Y-C, Huang C-Y, Liu C-C, Hour T-C, et al. Molecular markers in sex hormone pathway genes associated with the efficacy of androgen-deprivation therapy for prostate cancer. PLoS ONE. 2013;8:e54627.
Karunasinghe, N., Zhu, Y., Han, D. Y., Lange, K., Zhu, S., Wang, A., et al. Quality of life effects of androgen deprivation therapy in a prostate cancer cohort in New Zealand: can we minimize effects using a stratification based on the aldo-keto reductase family 1, member C3 rs12529 gene polymorphism? BMC Urol. 16, 48 (2016).
Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3:856–7.
Hearn JWD, Xie W, Nakabayashi M, Almassi N, Reichard CA, Pomerantz M, et al. Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 2018;4:558–62.
Chen WS, Alshalalfa M, Zhao SG, Liu Y, Mahal BA, Quigley DA, et al. Novel RB1-Loss transcriptomic signature is associated with poor clinical outcomes across cancer types. Clin Cancer Res. 2019;25:4290–9.
Chang K-H, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84.
Sabharwal N, Sharifi N. HSD3B1 genotypes conferring adrenal-restrictive and adrenal-permissive phenotypes in prostate cancer and beyond. Endocrinology. 2019;160:2180–8.
Hearn JWD, Sweeney C, Almassi N, Reichard CA, Reddy CA, Hobbs B, et al. HSD3B1 and overall survival (OS) in men with low-volume (LV) metastatic prostate cancer (PCa) treated with androgen deprivation therapy (ADT) or chemohormonal therapy in the CHAARTED Randomized trial. J Clin Oncol. 2019;37:5020–5020.
Chi K, Hotte SJ, Joshua AM, North S, Wyatt AW, Collins LL, et al. Treatment of mCRPC in the AR-axis-targeted therapy-resistant state. Ann Oncol. 2015;26:2044–56.
Grasso CS, Wu Y-M, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43.
Robinson D, Van Allen EM, Wu Y-M, Schultz N, Lonigro RJ, Mosquera J-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA. 2019;116:11428–36.
Acknowledgements
RA, NS, ES, and SGZ are supported by the Prostate Cancer Foundation. This work was supported in part with support from the National Cancer Institute (R01CA172382 and R01CA190289 to NS). Stand Up To Cancer-Prostate Cancer Foundation Prostate Cancer Dream Team Award (SU2C-AACR-DT0812 to EJS). Stand Up To Cancer (SU2C) is a division of the Entertainment Industry Foundation. This research grant was administered by the American Association for Cancer Research, the scientific partner of SU2C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SGZ has patents filed unrelated to this work with Decipher Biosciences and Celgene. A patent for 3β-hydroxysteroid dehydrogenase in steroid-dependent disease has been filed by Cleveland Clinic. JJA has performed consulting or held an advisory role with Astellas Pharma, Bayer, Merck, and Janssen Biotech, Inc. KNC has received grant support and honoraria from Astellas Pharma, Janssen, Sanofi, AstraZeneca, Bayer, Essa Pharma, Pfizer, and Roche. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, W.S., Feng, E.L., Aggarwal, R. et al. Germline polymorphisms associated with impaired survival outcomes and somatic tumor alterations in advanced prostate cancer. Prostate Cancer Prostatic Dis 23, 316–323 (2020). https://doi.org/10.1038/s41391-019-0188-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0188-4