Summary: A new injectable therapy that uses specially engineered molecules could help treat spinal cord injury.
Source: DOE
A new injectable therapy for spinal cord injuries uses specially engineered molecules that trigger a healing response in spinal cells.
The research team used X-ray characterization at the Advanced Photon Source (APS). This allowed the researchers to determine the structure of these molecules as they come together to form tiny fibers in a liquid solution.
Scientists can control the motion of these fibers, allowing the fibers to connect more effectively with cells in the spine.
The Impact
Hundreds of thousands of people worldwide suffer spinal injuries every year, often leading to paralysis. Scientists have been searching for decades for an effective treatment for these injuries.
This new injectable treatment reversed paralysis in mice after four weeks with just a single dose. If it does the same in humans, it could mean that people living with severe spinal injuries may have a hope of walking again.
The techniques and approaches to characterization with X-rays could also help develop other therapeutic approaches requiring insights on the molecular structure.
A critical portion of this research into a novel treatment for spinal injuries was conducted at the APS, a Department of Energy (DOE) Office of Science user facility at Argonne National Laboratory.
There, scientists from Northwestern University and the Air Force Research Laboratories used ultrabright X-ray beams to study the structure of the engineered molecules and how they behaved together in a solution.
Injected as a liquid, the molecules came together to form tiny fiber structures (called nanofibers) that surrounded the spinal cord.
In the APS studies, the researchers discovered that the motion of molecules within the nanofibers could be controlled by changing their chemical structure.
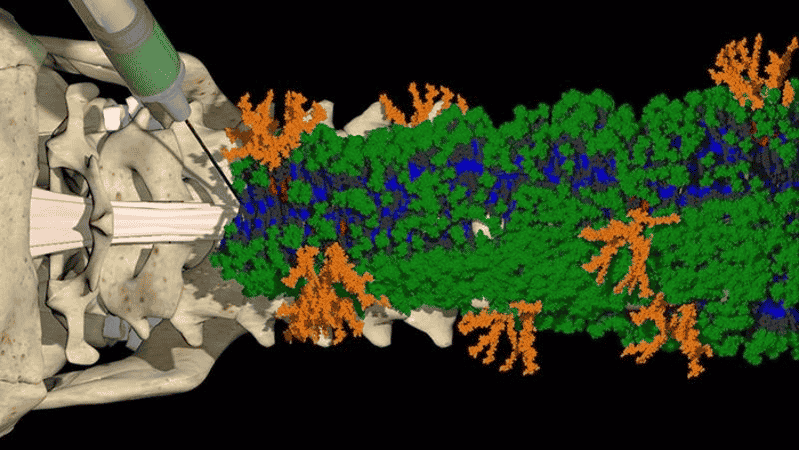
It turned out that molecules that moved most —“danced” more — were more likely to signal spinal cells via proteins called receptors, resulting in a more effective treatment.
Knowing the structure of the molecular matrix allowed researchers to tune the motion of the molecules.
By making the molecules “dance,” they were more likely to find and engage cellular receptors, triggering the cells to repair damaged neurons.
Funding
This research used resources of the APS, a Department of Energy (DOE) Office of Science user facility located at Argonne National Laboratory. Experimental work and simulations were supported by the Louis A. Simpson and Kimberly K. Querrey Center for Regenerative Nanomedicine at the Simpson Querrey Institute for BioNanotechnology. Analysis was supported by the Air Force Research Laboratory.
Experiments were supported by the National Institute on Neurological Disorders and Stroke, the National Institute on Aging, National Institutes for Health, the Les Turner ALS Foundation and the New York Stem Cell Foundation.
Portions of this work were performed at the DuPont-Northwestern-Dow Collaborative Access Team at the Advanced Photon Source (APS); this team is supported by Northwestern University, The Dow Chemical Company, and DuPont de Nemours, Inc.
About this spinal cord injury research news
Author: Michael Church
Source: DOE
Contact: Michael Church – DOE
Image: The image is credited to Northwestern University
Original Research: The findings will appear in Science






