Methods
Tool Overview
We used Excel 2010 (Microsoft Corporation, Redmond, WA, USA) to construct Anthrax Assist (online Technical Appendix 1, http://wwwnc.cdc.gov/EID/article/23/1/15-1787-Techapp1.xlsx). Anthrax Assist is composed of 3 linked models ( Table 1 ). The Epidemic-Curve model combines daily case counts with incubation distributions to project the future number and timing of symptomatic IA cases in a nonvaccinated population. The PEP Impact model estimates the potential decrease in the projected trajectory of future cases (output from the Epidemic-Curve model) resulting from a PEP dispensing campaign. The Healthcare Impact model uses the projected unmitigated or PEP-mitigated incidence curves to project the size and timing of peak healthcare utilization and associated patient outcomes. Users can readily change a number of input values to reflect a desired attack scenario or response strategy ( Table 2 ). To illustrate the models, we developed an attack scenario and used it to evaluate estimates resulting from various outbreak detection scenarios (using 1, 2, or 3 days of initial case count data) and PEP response strategies ( Table 3 ).
Calculations
Epidemic-curve Model. We base our IA incubation distribution on the Wilkening model, which plots the probability of becoming symptomatic over a 60-day period for a given infectious dose of B. anthracis spores (online Technical Appendix 2, http://wwwnc.cdc.gov/EID/article/23/1/15-1787-Techapp2.pdf).[13] We combine this incubation probability distribution with the number of detected IA cases at a given time to calculate the total projected number of ill persons (final case count [FCC]) by using the following equation:
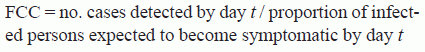
where t is the number of days from the date of the first symptomatic case to the time of analysis. The numerator is obtained through public health disease surveillance, and the denominator is obtained from the incubation probability distribution. We then generate an epidemic curve by distributing the FCC over each day of the outbreak according to the incubation probability distribution.
We assume a single, localized release that causes near-simultaneous population exposure. Because public health authorities will probably not know the average inhaled spore dose among affected persons, we designed the model to calculate a range of plausible outbreak sizes from a range of spores inhaled per person. To illustrate the model, we used a median value of 360 spores/person (range 1–8,000), resulting in a median incubation period of 6.9 days (range 10.3–5.0) (Table 2; online Technical Appendix 2).
PEP Impact Model. The PEP Impact model uses median projected daily case counts (output from the Epidemic Curve model) to estimate the potential effects of a PEP campaign. This effect is calculated as the product of the number of persons who become symptomatic on any given day t; the effectiveness of PEP on day t (which is a product of antimicrobial efficacy and adherence); and the probability that an infected, asymptomatic person receives antimicrobial prophylaxis on or before day t. We calculate the probability that a person receives PEP on day t by multiplying the PEP uptake (proportion of persons seeking antimicrobial drugs) by the daily antimicrobial dispensing throughput and then dividing by the population targeted for PEP (Table 2). The FCC with a PEP campaign is the sum of detected cases and daily PEP-mitigated case count projections. We express PEP effect as both a difference measure (cases averted) and as a proportion (cases averted divided by the unmitigated FCC). We assume that symptomatic persons seeking PEP are referred for medical treatment and do not receive PEP.[21] We further assume that all of the population suspected to be exposed would be targeted for PEP because there is no definitive PEP triage process for IA beyond exposure risk (Table 2).
In accordance with US CRI guidelines, we assume that PEP dispensing is completed in 2 (range 1–2) days after the decision to initiate PEP.[9] Following SteelFisher et al., we also assume that of the population targeted to receive PEP, 65% (range 40%–90%) actually start taking PEP.[11] Everyone starting PEP is assumed to fully adhere to the regimen on the first day. After that, adherence decreases linearly to 40% (range 25%–80%) at the conclusion of the event (online Technical Appendix 2).[18] Last, we assumed 90% (range 10%–90%) antimicrobial drug efficacy and that this level of protection is achieved 1 day after initiation of the regimen[15,16] (Table 2).
Healthcare Impact Model. To calculate the demand for medical care, we used a compartmental model (based on one reported by Zaric et al.) and used the review of IA cases by Holty et al. to select the rates of patients' transitions through illness stages[6,19] (Figure 1; online Technical Appendix 2). This model is used to calculate daily patients initiating treatment, peak daily treatment caseload (i.e., census of hospitalized patients receiving treatment for IA), and the day of peak treatment caseload.
Figure 1.
Anthrax Assist model disease stages, intervention states, and transitions. Persons begin in the top Incubation state and may transition via the numbered arrows from one state to another until they eventually reach an outcome state (doubled-walled boxes). All persons with untreated infection will progress to deceased. Recovery is possible only through effective oral PEP (averted case) or anthrax-specific treatment (recovered). Transitions are governed by the 3 Anthrax Assist models as follows: Epidemic-Curve model, transition 1; PEP Impact model, transitions 2 and 3; Healthcare Impact model, transitions 4–11. Suspected, but Not Actually Exposed cases are shown here because of their role in diluting the incubating population seeking PEP (dashed transition arrow). PEP and Treatment queues (dashed outline boxes) are depicted to reflect the necessary interactions persons must have with the public health and healthcare systems to transition between treatment states. PEP, postexposure prophylaxis.
In this model, medical intervention is required for recovery from symptomatic IA, and only patients with fulminant disease can die. We define treatment effectiveness as the percentage of patients who recover after receiving some type of medical intervention and pattern it after the 2001 US IA events. As such, treatment is 4 times more effective when started in the prodromal (80%), rather than fulminant (20%), stage of illness (Table 2). However, the probability that a patient in the fulminant stage seeks healthcare (95%) is roughly twice that for someone in the prodromal stage (40%).[22] In addition, we varied the likelihood that any patient seeks healthcare by the timing of public health messaging regarding screening and treatment recommendations. We assume that the proportion of persons in the prodromal stage who seek care would double as a result of widespread media attention (80% vs. 40%)[2] (Table 2). Last, we assume treatment effectiveness values based on full availability of medical countermeasures and resources at the time of treatment and no delay in access to care once sought (Table 2).
During the 2001 US IA event, treatment duration was highly associated with treatment outcomes.[22] Thus, for those who recover, we assume a normal distribution with a mean of 18 (SD 3) treatment days from the date of transition to the fulminant stage of illness or from the sixth day of prodromal illness for patients whose illness does not progress to the fulminant stage. For patients who eventually recover from fulminant illness (in treated and yet-nontreated populations), we assume a 20% transition each day so that all have transitioned to the fulminant stage after 5 days in the prodromal stage. Among those who eventually die, half transition to the fulminant stage on the first day of symptoms and the other half on the next day. When treatment is not sought, we assume that death occurs on the same day as the transition to fulminant illness.
Scenarios
To illustrate use of the models, we created an attack case series scenario patterned after the 1979 Sverdlovsk, USSR, event, in which at least 70 people died of IA after accidental aerosol release of B. anthracis spores from a bioweapons facility (Table 2).[12] We created this Sverdlovsk-like case series by multiplying each day's case count from the Sverdlovsk event by 10, resulting in a 40-day, 700-patient case series (online Technical Appendix 2).
To illustrate the accuracy of the Anthrax Assist FCC projections under realistic conditions of limited reported case data in the first days of an event, we first ran the Epidemic-Curve portion of Anthrax Assist by using only the first 3 days of case data as input (20, 10, and 70 cases, respectively), then by using 2 days of case data, and then only the first day's cases. To examine the effect of the number of days of case data on the accuracy of our FCC projection, we also incrementally added a day of case data, beyond the first 3 days, until the projection was within 10% of the true FCC.
Next, to evaluate prophylaxis response options, we developed 4 PEP scenarios by varying components of the PEP campaign implementation (logistics) and the public response to the campaign (utilization) (Table 3). Scenario 1 (no PEP) is an event without a PEP campaign. Scenario 2 (ideal) is an event wherein early detection of the event (e.g., through biosensors) and positive public perception results in a 1-day campaign starting 1 day after detection, 90% uptake, and 80% adherence at the event's conclusion. Scenario 3 (practical) is an event in which PEP dispensing logistics follow current public health guidance and PEP utilization is based on data from the 2001 US IA event, resulting in a 2-day campaign starting 2 days after detection, 65% uptake, and 40% adherence at the event conclusion. Scenario 4 (constrained) is an event in which logistics hurdles (e.g. staffing shortages, traffic congestion 3,23 ) and poor public perception impede rapid PEP coverage, resulting in a 4-day campaign starting 2 days after detection, 40% uptake, and 25% adherence at event conclusion. Hereafter, the baseline scenario comprises PEP scenario 3 and the Healthcare Impact model values in Table 2.
Sensitivity Analyses
We conducted 2 sensitivity analyses. We first evaluated the influence of individual PEP-related parameters on outputs from the models as follows: prophylaxis campaign duration of 1–6 days at full throughput capacity, delay of 3–6 days until PEP campaign starts, a range of 15%–90% for PEP uptake, a range of 10%–90% for antimicrobial efficacy, and a range of 15%–90% for adherence to the regimen at the conclusion of the event. These ranges encompass reported values.[3,4,11,18,24]
In our second sensitivity analysis, we altered the Epidemic-Curve model inputs used in the baseline attack scenario to illustrate potential data limitations and surveillance inaccuracies that might occur during an actual event. Doing so involved comparing estimates using the full complement of the initial 3 days of case data with a scenario in which 60% of cases are reported. This level of underreporting represents the plausible difficulties often encountered when initially collecting outbreak data.
Emerging Infectious Diseases. 2017;23(1):46-55. © 2017 Centers for Disease Control and Prevention (CDC)







