Abstract
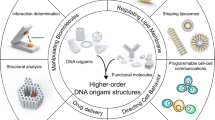
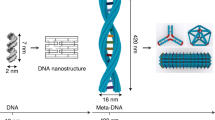
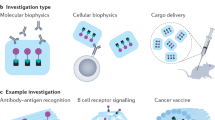
Biological materials are self-assembled with near-atomic precision in living cells, whereas synthetic 3D structures generally lack such precision and controllability. Recently, DNA nanotechnology, especially DNA origami technology, has been useful in the bottom-up fabrication of well-defined nanostructures ranging from tens of nanometres to sub-micrometres. In this Primer, we summarize the methodologies of DNA origami technology, including origami design, synthesis, functionalization and characterization. We highlight applications of origami structures in nanofabrication, nanophotonics and nanoelectronics, catalysis, computation, molecular machines, bioimaging, drug delivery and biophysics. We identify challenges for the field, including size limits, stability issues and the scale of production, and discuss their possible solutions. We further provide an outlook on next-generation DNA origami techniques that will allow in vivo synthesis and multiscale manufacturing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Seeman, N. C. Nucleic-acid junctions and lattices. J. Theor. Biol. 99, 237–247 (1982). This study, as the beginning of DNA nanotechnology, theoretically predicts immobile branched DNA junctions and their 3D assemblies.
Fu, T. J. & Seeman, N. C. DNA double-crossover molecules. Biochemistry 32, 3211–3220 (1993).
LaBean, T. H. et al. Construction, analysis, ligation, and self-assembly of DNA triple crossover complexes. J. Am. Chem. Soc. 122, 1848–1860 (2000).
Yan, H., Park, S. H., Finkelstein, G., Reif, J. H. & LaBean, T. H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 301, 1882–1884 (2003).
He, Y. et al. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 452, 198–201 (2008).
Seeman, N. C. & Sleiman, H. F. DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2017). This review provides a more comprehensive view of DNA nanotechnology, covering branches other than DNA origami.
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006). This study is the first demonstration of DNA origami.
Douglas, S. M. et al. Rapid prototyping of 3D DNA origami shapes with caDNAno. Nucleic Acids Res. 37, 5001–5006 (2009). This study provides a 1G visual DNA origami design tool that is an important catalyst for this field.
Jun, H. et al. Automated sequence design of 3D polyhedral wireframe DNA origami with honeycomb edges. ACS Nano 13, 2083–2093 (2019).
Jun, H., Wang, X., Bricker, W. P. & Bathe, M. Automated sequence design of 2D wireframe DNA origami with honeycomb edges. Nat. Commun. 10, 5419 (2019).
Dunn, K. E. et al. Guiding the folding pathway of DNA origami. Nature 525, 82–86 (2015). This study explains why DNA origami can generate desired shapes with high yields, from the perspective of cooperativity in folding.
Schneider, F., Möritz, N. & Dietz, H. The sequence of events during folding of a DNA origami. Sci. Adv. 5, eaaw1412 (2019).
Qian, L. et al. Analogic China map constructed by DNA. Chin. Sci. Bull. 51, 2973 (2006).
Andersen, E. S. et al. DNA origami design of dolphin-shaped structures with flexible tails. ACS Nano 2, 1213–1218 (2008).
Benson, E. et al. DNA rendering of polyhedral meshes at the nanoscale. Nature 523, 441–444 (2015). This paper presents a general and automated design method based on graph theory for building arbitrary polygonal structures that are difficult to realize using previous approaches.
Sun, W. et al. Casting inorganic structures with DNA molds. Science 346, 1258361 (2014).
Douglas, S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009). This study demonstrates the building of 3D DNA origami structures in form of honeycomb lattices.
Dietz, H., Douglas, S. M. & Shih, W. M. Folding DNA into twisted and curved nanoscale shapes. Science 325, 725–730 (2009). This study demonstrates the construction of DNA origami shapes with quantitative twists and curvatures, by rational insertions and deletions of base pairs into and from the DNA bundles.
Han, D. et al. DNA origami with complex curvatures in three-dimensional space. Science 332, 342–346 (2011).
Wagenbauer, K. F., Sigl, C. & Dietz, H. Gigadalton-scale shape-programmable DNA assemblies. Nature 552, 78–83 (2017).
Tikhomirov, G., Petersen, P. & Qian, L. Fractal assembly of micrometre-scale DNA origami arrays with arbitrary patterns. Nature 552, 67–71 (2017).
Yao, G. et al. Meta-DNA structures. Nat. Chem. 12, 1067–1075 (2020).
Han, D. et al. Single-stranded DNA and RNA origami. Science 358, eaao2648 (2017).
Geary, C., Rothemund, P. W. K. & Andersen, E. S. A single-stranded architecture for cotranscriptional folding of RNA nanostructures. Science 345, 799–804 (2014). This study demonstrates the potential of synthesizing origami-like RNA nanostructures in biological systems.
Zhang, D. Y. & Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 3, 103–113 (2011).
Gerling, T., Wagenbauer, K. F., Neuner, A. M. & Dietz, H. Dynamic DNA devices and assemblies formed by shape-complementary, non-base pairing 3D components. Science 347, 1446–1452 (2015). This study demonstrates that DNA origami structures can be further assembled into higher order yet dynamically reconfigurable structures based on shape complementarity rather than base pairing, which provides an approach to overcome the size limit of DNA origami.
Ge, Z., Gu, H., Li, Q. & Fan, C. Concept and development of framework nucleic acids. J. Am. Chem. Soc. 140, 17808–17819 (2018).
Liu, X. et al. Complex silica composite nanomaterials templated with DNA origami. Nature 559, 593–598 (2018).
Perrault, S. D. & Shih, W. M. Virus-inspired membrane encapsulation of DNA nanostructures to achieve in vivo stability. ACS Nano 8, 5132–5140 (2014).
Agarwal, N. P., Matthies, M., Gur, F. N., Osada, K. & Schmidt, T. L. Block copolymer micellization as a protection strategy for DNA origami. Angew. Chem. Int. Ed. 56, 5460–5464 (2017).
Liu, N. & Liedl, T. DNA-assembled advanced plasmonic architectures. Chem. Rev. 118, 3032–3053 (2018). This review focuses on the progress of using DNA nanostructures as scaffolds for creating plasmonic architectures, which is an important field for nanophotonic applications.
Maune, H. T. et al. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nat. Nanotechnol. 5, 61–66 (2010).
Jin, Z. et al. Metallized DNA nanolithography for encoding and transferring spatial information for graphene patterning. Nat. Commun. 4, 1663 (2013).
Jungmann, R. et al. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods 11, 313–318 (2014).
Nicoli, F. et al. Directional photonic wire mediated by homo-Förster resonance energy transfer on a DNA origami platform. ACS Nano 11, 11264–11272 (2017).
Dutta, P. K. et al. DNA-directed artificial light-harvesting antenna. J. Am. Chem. Soc. 133, 11985–11993 (2011).
Yurke, B., Turberfield, A. J., Mills, A. P., Simmel, F. C. & Neumann, J. L. A DNA-fuelled molecular machine made of DNA. Nature 406, 605–608 (2000).
Lu, C. H. & Willner, I. Stimuli-responsive DNA-functionalized nano-/microcontainers for switchable and controlled release. Angew. Chem. Int. Ed. 54, 12212–12235 (2015).
Jiang, Q., Liu, S., Liu, J., Wang, Z. & Ding, B. Rationally designed DNA origami nanomaterials for drug delivery in vivo. Adv. Mater. 31, e1804785 (2019). This review focuses on the progress in drug delivery application based on DNA origami.
Li, S. et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 36, 258–264 (2018).
Li, J., Green, A. A., Yan, H. & Fan, C. Engineering nucleic acid structures for programmable molecular circuitry and intracellular biocomputation. Nat. Chem. 9, 1056–1067 (2017).
Thubagere, A. J. et al. A cargo-sorting DNA robot. Science 357, eaan6558 (2017).
Chao, J. et al. Solving mazes with single-molecule DNA navigators. Nat. Mater. 18, 273–279 (2019).
Kuzyk, A. et al. A light-driven three-dimensional plasmonic nanosystem that translates molecular motion into reversible chiroptical function. Nat. Commun. 7, 10591 (2016).
Zhang, F. et al. Complex wireframe DNA origami nanostructures with multi-arm junction vertices. Nat. Nanotechnol. 10, 779–784 (2015).
Wagenbauer, K. F. et al. How we make DNA origami. ChemBioChem 18, 1873–1885 (2017).
Veneziano, R. et al. Designer nanoscale DNA assemblies programmed from the top down. Science 352, 1534 (2016).
Benson, E. et al. Computer-aided production of scaffolded DNA nanostructures from flat sheet meshes. Angew. Chem. Int. Ed. 55, 8869–8872 (2016).
Jun, H. et al. Autonomously designed free-form 2D DNA origami. Sci. Adv. 5, eaav0655 (2019).
Huang, C.-M., Kucinic, A., Johnson, J. A., Su, H.-J. & Castro, C. E. Integrating computer-aided engineering and computer-aided design for DNA assemblies. Preprint at bioRxiv https://doi.org/10.1101/2020.05.28.119701 (2020).
Jun, H., Wang, X., Bricker, W. P., Jackson, S. & Bathe, M. Rapid prototyping of wireframe scaffolded DNA origami using ATHENA. Preprint at bioRxiv https://doi.org/10.1101/2020.02.09.940320 (2020).
de Llano, E. et al. Adenita: interactive 3D modelling and visualization of DNA nanostructures. Nucleic Acids Res. 48, 8269–8275 (2020).
Yoo, J. & Aksimentiev, A. In situ structure and dynamics of DNA origami determined through molecular dynamics simulations. Proc. Natl Acad. Sci. USA 110, 20099–20104 (2013).
Göpfrich, K. et al. Large-conductance transmembrane porin made from DNA origami. ACS Nano 10, 8207–8214 (2016).
Savelyev, A. & Papoian, G. A. Molecular renormalization group coarse-graining of polymer chains: application to double-stranded DNA. Biophys. J. 96, 4044–4052 (2009).
Pan, K. et al. Lattice-free prediction of three-dimensional structure of programmed DNA assemblies. Nat. Commun. 5, 5578 (2014).
Nakano, S. Nucleic acid duplex stability: influence of base composition on cation effects. Nucleic Acids Res. 27, 2957–2965 (1999).
Wohlert, J., den Otter, W. K., Edholm, O. & Briels, W. J. Free energy of a trans-membrane pore calculated from atomistic molecular dynamics simulations. J. Chem. Phys. 124, 154905 (2006).
Reshetnikov, R. V. et al. A coarse-grained model for DNA origami. Nucleic Acids Res. 46, 1102–1112 (2018).
Poppleton, E. et al. Design, optimization and analysis of large DNA and RNA nanostructures through interactive visualization, editing and molecular simulation. Nucleic Acids Res. 48, e72 (2020).
Doye, J. P. K. et al. Coarse-graining DNA for simulations of DNA nanotechnology. Phys. Chem. Chem. Phys. 15, 20395–20414 (2013).
Snodin, B. E. K. et al. Introducing improved structural properties and salt dependence into a coarse-grained model of DNA. J. Chem. Phys. 142, 234901 (2015).
Maffeo, C. & Aksimentiev, A. MrDNA: a multi-resolution model for predicting the structure and dynamics of DNA systems. Nucleic Acids Res. 48, 5135–5146 (2020).
Veneziano, R. et al. In vitro synthesis of gene-length single-stranded DNA. Sci. Rep. 8, 6548 (2018).
Zhang, H. et al. Folding super-sized DNA origami with scaffold strands from long-range PCR. Chem. Commun. 48, 6405–6407 (2012).
Erkelenz, M. et al. A facile method for preparation of tailored scaffolds for DNA origami. Small 10, 73–77 (2014).
Marchi, A. N., Saaem, I., Vogen, B. N., Brown, S. & LaBean, T. H. Toward larger DNA origami. Nano Lett. 14, 5740–5747 (2014).
Nafisi, P. M., Aksel, T. & Douglas, S. M. Construction of a novel phagemid to produce custom DNA origami scaffolds. Synth. Biol. 3, ysy015 (2018).
Ke, Y., Bellot, G., Voigt, N. V., Fradkov, E. & Shih, W. M. Two design strategies for enhancement of multilayer-DNA origami folding: underwinding for specific intercalator rescue and staple-break positioning. Chem. Sci. 3, 2587–2597 (2012).
Martin, T. G. & Dietz, H. Magnesium-free self-assembly of multi-layer DNA objects. Nat. Commun. 3, 1103 (2012).
Kielar, C. et al. On the stability of DNA origami nanostructures in low-magnesium buffers. Angew. Chem. Int. Ed. 57, 9470–9474 (2018).
Hahn, J., Wickham, S. F., Shih, W. M. & Perrault, S. D. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano 8, 8765–8775 (2014).
Castro, C. E. et al. A primer to scaffolded DNA origami. Nat. Methods 8, 221–229 (2011).
Hong, F., Zhang, F., Liu, Y. & Yan, H. DNA origami: scaffolds for creating higher order structures. Chem. Rev. 117, 12584–12640 (2017).
Schickinger, M., Zacharias, M. & Dietz, H. Tethered multifluorophore motion reveals equilibrium transition kinetics of single DNA double helices. Proc. Natl Acad. Sci. USA 115, E7512–E7521 (2018).
Sobczak, J.-P. J., Martin, T. G., Gerling, T. & Dietz, H. Rapid folding of DNA into nanoscale shapes at constant temperature. Science 338, 1458–1461 (2012).
Liu, W., Zhong, H., Wang, R. & Seeman, N. C. Crystalline two-dimensional DNA origami arrays. Angew. Chem. Int. Ed. 50, 264–267 (2011).
Kocabey, S. et al. Membrane-assisted growth of DNA origami nanostructure arrays. ACS Nano 9, 3530–3539 (2015).
Suzuki, Y., Endo, M. & Sugiyama, H. Lipid-bilayer-assisted two-dimensional self-assembly of DNA origami nanostructures. Nat. Commun. 6, 1–9 (2015).
Rajendran, A., Endo, M., Katsuda, Y., Hidaka, K. & Sugiyama, H. Photo-cross-linking-assisted thermal stability of DNA origami structures and its application for higher-temperature self-assembly. J. Am. Chem. Soc. 133, 14488–14491 (2011).
Woo, S. & Rothemund, P. W. K. Programmable molecular recognition based on the geometry of DNA nanostructures. Nat. Chem. 3, 620–627 (2011).
Zhang, T. et al. 3D DNA origami crystals. Adv. Mater. 30, 1800273 (2018).
Bellot, G., McClintock, M. A., Lin, C. & Shih, W. M. Recovery of intact DNA nanostructures after agarose gel-based separation. Nat. Methods 8, 192–194 (2011).
Shaw, A., Benson, E. & Högberg, B. Purification of functionalized DNA origami nanostructures. ACS Nano 9, 4968–4975 (2015).
Stahl, E., Martin, T. G., Praetorius, F. & Dietz, H. Facile and scalable preparation of pure and dense DNA origami solutions. Angew. Chem. Int. Ed. 53, 12735–12740 (2014).
Lin, C., Perrault, S. D., Kwak, M., Graf, F. & Shih, W. M. Purification of DNA origami nanostructures by rate-zonal centrifugation. Nucleic Acids Res. 41, e40–e40 (2013).
Wagenbauer, K. F., Wachauf, C. H. & Dietz, H. Quantifying quality in DNA self-assembly. Nat. Commun. 5, 3691 (2014).
Sacca, B. et al. Reversible reconfiguration of DNA origami nanochambers monitored by single-molecule FRET. Angew. Chem. Int. Ed. 54, 3592–3597 (2015).
Andersen, E. S. et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 459, 73–76 (2009). This work presents a DNA origami container whose lid can be dynamically opened via toehold-mediated strand displacement, which inspires later studies on controllable cargo release.
Grossi, G., Jepsen, M. D. E., Kjems, J. & Andersen, E. S. Control of enzyme reactions by a reconfigurable DNA nanovault. Nat. Commun. 8, 992 (2017).
Wei, X., Nangreave, J. & Liu, Y. Uncovering the self-assembly of DNA nanostructures by thermodynamics and kinetics. Acc. Chem. Res. 47, 1861–1870 (2014).
Wei, X., Nangreave, J., Jiang, S., Yan, H. & Liu, Y. Mapping the thermal behavior of DNA origami nanostructures. J. Am. Chem. Soc. 135, 6165–6176 (2013).
Opherden, L., Oertel, J., Barkleit, A., Fahmy, K. & Keller, A. Paramagnetic decoration of DNA origami nanostructures by Eu3+ coordination. Langmuir 30, 8152–6159 (2014).
Schreiber, R. et al. Chiral plasmonic DNA nanostructures with switchable circular dichroism. Nat. Commun. 4, 2948 (2013).
Rajendran, A., Endo, M. & Sugiyama, H. Single-molecule analysis using DNA origami. Angew. Chem. Int. Ed. 51, 874–890 (2012).
Birkedal, V. et al. Single molecule microscopy methods for the study of DNA origami structures. Microsc. Res. Tech. 74, 688–698 (2011).
Jungmann, R., Scheible, M. & Simmel, F. C. Nanoscale imaging in DNA nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 4, 66–81 (2012).
Wah, J. L., David, C., Rudiuk, S., Baigl, D. & Estevez-Torres, A. Observing and controlling the folding pathway of DNA origami at the nanoscale. ACS Nano 10, 1978–1987 (2016).
Kosinski, R. et al. Sites of high local frustration in DNA origami. Nat. Commun. 10, 1061 (2019).
Song, J. et al. Reconfiguration of DNA molecular arrays driven by information relay. Science 357, eaan3377 (2017).
Sannohe, Y., Endo, M., Katsuda, Y., Hidaka, K. & Sugiyama, H. Visualization of dynamic conformational switching of the G-quadruplex in a DNA nanostructure. J. Am. Chem. Soc. 132, 16311–16313 (2010).
Endo, M., Katsuda, Y., Hidaka, K. & Sugiyama, H. A versatile DNA nanochip for direct analysis of DNA base-excision repair. Angew. Chem. Int. Ed. 49, 9412–9416 (2010).
Ramakrishnan, S. et al. Real-time observation of superstructure-dependent DNA origami digestion by DNase I using high-speed atomic force microscopy. ChemBioChem 20, 2818–2823 (2019).
Bai, X. C., Martin, T. G., Scheres, S. H. & Dietz, H. Cryo-EM structure of a 3D DNA origami object. Proc. Natl Acad. Sci. USA 109, 20012–20017 (2012).
Sharonov, A. & Hochstrasser, R. M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl Acad. Sci. USA 103, 18911–18916 (2006).
Jungmann, R. et al. Single-molecule kinetics and super-resolution microscopy by fluorescence imaging of transient binding on DNA origami. Nano Lett. 10, 4756–4761 (2010).
Schnitzbauer, J., Strauss, M. T., Schlichthaerle, T., Schueder, F. & Jungmann, R. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 12, 1198–1228 (2017).
Lund, K. et al. Molecular robots guided by prescriptive landscapes. Nature 465, 206–210 (2010).
Zhao, Z. et al. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 7, 10619 (2016).
Kopperger, E. et al. A self-assembled nanoscale robotic arm controlled by electric fields. Science 359, 296–301 (2018).
Thomsen, R. P. et al. A large size-selective DNA nanopore with sensing applications. Nat. Commun. 10, 5655 (2019).
Shrestha, P. et al. Mechanical properties of DNA origami nanoassemblies are determined by Holliday junction mechanophores. Nucleic Acids Res. 44, 6574–6582 (2016).
Engel, M. C. et al. Force-induced unravelling of DNA origami. ACS Nano 12, 6734–6747 (2018).
Shrestha, P. et al. Confined space facilitates G-quadruplex formation. Nat. Nanotechnol. 12, 582–588 (2017).
Iwaki, M., Wickham, S. F., Ikezaki, K., Yanagida, T. & Shih, W. M. A programmable DNA origami nanospring that reveals force-induced adjacent binding of myosin VI heads. Nat. Commun. 7, 13715 (2016).
Madsen, M. & Gothelf, K. V. Chemistries for DNA nanotechnology. Chem. Rev. 119, 6384–6458 (2019).
Tian, Y. et al. Prescribed nanoparticle cluster architectures and low-dimensional arrays built using octahedral DNA origami frames. Nat. Nanotechnol. 10, 637–644 (2015).
Kuzyk, A. et al. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 483, 311–314 (2012).
Hartl, C. et al. Position accuracy of gold nanoparticles on DNA origami structures studied with small-angle X-ray scattering. Nano Lett. 18, 2609–2615 (2018).
Kuzyk, A. et al. Reconfigurable 3D plasmonic metamolecules. Nat. Mater. 13, 862–866 (2014).
Pal, S. et al. DNA directed self-assembly of anisotropic plasmonic nanostructures. J. Am. Chem. Soc. 133, 17606–17609 (2011).
Zhang, Y. et al. Nanoparticle-assisted alignment of carbon nanotubes on DNA origami. Angew. Chem. Int. Ed. 59, 4892–4896 (2020).
Pei, H. et al. Organizing end-site-specific SWCNTs in specific loci using DNA. J. Am. Chem. Soc. 141, 11923–11928 (2019).
Liu, W. Y., Halverson, J., Tian, Y., Tkachenko, A. V. & Gang, O. Self-organized architectures from assorted DNA-framed nanoparticles. Nat. Chem. 8, 867–873 (2016).
Liu, W. et al. Diamond family of nanoparticle superlattices. Science 351, 582–586 (2016). This study creates a diamond superlattice via the constrained packing of triangular binding footprints of DNA origami tetrahedra.
Tian, Y. et al. Lattice engineering through nanoparticle–DNA frameworks. Nat. Mater. 15, 654–661 (2016).
Zhan, P. et al. Reconfigurable three-dimensional gold nanorod plasmonic nanostructures organized on DNA origami tripod. ACS Nano 11, 1172–1179 (2017).
Liu, J. F. et al. Metallization of branched DNA origami for nanoelectronic circuit fabrication. ACS Nano 5, 2240–2247 (2011).
Pilo-Pais, M., Goldberg, S., Samano, E., Labean, T. H. & Finkelstein, G. Connecting the nanodots: programmable nanofabrication of fused metal shapes on DNA templates. Nano Lett. 11, 3489–3492 (2011).
Schreiber, R. et al. DNA origami-templated growth of arbitrarily shaped metal nanoparticles. Small 7, 1795–1799 (2011).
Jia, S. et al. Programming DNA origami patterning with non-canonical DNA-based metallization reactions. Nat. Commun. 10, 5597 (2019).
Shang, Y. et al. Site-specific synthesis of silica nanostructures on DNA origami templates. Adv. Mater. 32, 2000294 (2020).
Bayrak, T. et al. DNA-mold templated assembly of conductive gold nanowires. Nano Lett. 18, 2116–2123 (2018).
Nguyen, L., Doblinger, M., Liedl, T. & Heuer-Jungemann, A. DNA origami-templated silica growth by sol-gel chemistry. Angew. Chem. Int. Ed. 58, 912–916 (2019).
Zhang, Y. et al. Transfer of two-dimensional oligonucleotide patterns onto stereocontrolled plasmonic nanostructures through DNA origami-based nanoimprinting lithography. Angew. Chem. Int. Ed. 55, 8036–8040 (2016).
Gallego, I. et al. DNA origami-driven lithography for patterning on gold surfaces with sub-10 nm resolution. Adv. Mater. 29, 1603233 (2017).
Tian, C. et al. DNA nanostructures-mediated molecular imprinting lithography. ACS Nano 11, 227–238 (2017).
Hung, A. M. et al. Large-area spatially ordered arrays of gold nanoparticles directed by lithographically confined DNA origami. Nat. Nanotechnol. 5, 121–126 (2010).
Gopinath, A., Miyazono, E., Faraon, A. & Rothemund, P. W. Engineering and mapping nanocavity emission via precision placement of DNA origami. Nature 535, 401–405 (2016).
Kershner, R. J. et al. Placement and orientation of individual DNA shapes on lithographically patterned surfaces. Nat. Nanotechnol. 4, 557–561 (2009).
Hentschel, M., Schäferling, M., Duan, X., Giessen, H. & Liu, N. Chiral plasmonics. Sci. Adv. 3, e1602735 (2017).
Fang, W. et al. Quantizing single-molecule surface-enhanced Raman scattering with DNA origami metamolecules. Sci. Adv. 5, eaau4506 (2019).
Weller, L. et al. Gap-dependent coupling of Ag–Au nanoparticle heterodimers using DNA origami-based self-assembly. ACS Photonics 3, 1589–1595 (2016).
Roller, E. M. et al. Hotspot-mediated non-dissipative and ultrafast plasmon passage. Nat. Phys. 13, 761–765 (2017).
Vogele, K. et al. Self-assembled active plasmonic waveguide with a peptide-based thermomechanical switch. ACS Nano 10, 11377–11384 (2016).
Shen, X. et al. Rolling up gold nanoparticle-dressed DNA origami into three-dimensional plasmonic chiral nanostructures. J. Am. Chem. Soc. 134, 146–149 (2012).
Urban, M. J. et al. Plasmonic toroidal metamolecules assembled by DNA origami. J. Am. Chem. Soc. 138, 5495–5498 (2016).
Shen, X. B. et al. Three-dimensional plasmonic chiral tetramers assembled by DNA origami. Nano Lett. 13, 2128–2133 (2013).
Lan, X. et al. Bifacial DNA origami-directed discrete, three-dimensional, anisotropic plasmonic nanoarchitectures with tailored optical chirality. J. Am. Chem. Soc. 135, 11441–11444 (2013).
Lan, X. et al. Au nanorod helical superstructures with designed chirality. J. Am. Chem. Soc. 137, 457–462 (2015).
Zhou, C., Duan, X. Y. & Liu, N. A plasmonic nanorod that walks on DNA origami. Nat. Commun. 6, 8102 (2015).
Urban, M. J. et al. Gold nanocrystal-mediated sliding of doublet DNA origami filaments. Nat. Commun. 9, 1454 (2018).
Zhou, X. et al. Efficient long-range, directional energy transfer through DNA-templated dye aggregates. J. Am. Chem. Soc. 141, 8473–8481 (2019).
Samanta, A., Zhou, Y., Zou, S., Yan, H. & Liu, Y. Fluorescence quenching of quantum dots by gold nanoparticles: a potential long range spectroscopic ruler. Nano Lett. 14, 5052–5057 (2014).
Thacker, V. V. et al. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat. Commun. 5, 3448 (2014).
Acuna, G. P. et al. Fluorescence enhancement at docking sites of DNA-directed self-assembled nanoantennas. Science 338, 506–510 (2012).
Puchkova, A. et al. DNA origami nanoantennas with over 5000-fold fluorescence enhancement and single-molecule detection at 25 μM. Nano Lett. 15, 8354–8359 (2015).
Zhao, M. et al. DNA-directed nanofabrication of high-performance carbon nanotube field-effect transistors. Science 368, 878–881 (2020).
Fu, J. L. et al. DNA-scaffolded proximity assembly and confinement of multienzyme reactions. Top. Curr. Chem. 378, 38 (2020).
Niemeyer, C. M., Koehler, J. & Wuerdemann, C. DNA-directed assembly of bienzymic complexes from in vivo biotinylated NAD(P)H:FMN oxidoreductase and luciferase. ChemBioChem 3, 242–245 (2002).
Wilner, O. I. et al. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotechnol. 4, 249–254 (2009).
Fu, J., Liu, M., Liu, Y., Woodbury, N. W. & Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 134, 5516–5519 (2012).
Liu, M. et al. A three-enzyme pathway with an optimised geometric arrangement to facilitate substrate transfer. ChemBioChem 17, 1097–1101 (2016).
Fu, J. et al. Assembly of multienzyme complexes on DNA nanostructures. Nat. Protoc. 11, 2243–2273 (2016).
Zhang, Y., Tsitkov, S. & Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade. Nat. Commun. 7, 13982 (2016).
Kuzmak, A., Carmali, S., von Lieres, E., Russell, A. J. & Kondrat, S. Can enzyme proximity accelerate cascade reactions? Sci. Rep. 9, 455 (2019).
Sprengel, A. et al. Tailored protein encapsulation into a DNA host using geometrically organized supramolecular interactions. Nat. Commun. 8, 14472 (2017).
Kohman, R. E., Cha, S. S., Man, H. Y. & Han, X. Light-triggered release of bioactive molecules from DNA nanostructures. Nano Lett. 16, 2781–2785 (2016).
Fu, Y. et al. Single-step rapid assembly of DNA origami nanostructures for addressable nanoscale bioreactors. J. Am. Chem. Soc. 135, 696–702 (2013).
Linko, V., Eerikainen, M. & Kostiainen, M. A. A modular DNA origami-based enzyme cascade nanoreactor. Chem. Commun. 51, 5351–5354 (2015).
Zhang, Y. F. & Hess, H. Toward rational design of high-efficiency enzyme cascades. ACS Catal. 7, 6018–6027 (2017).
Adleman, L. M. Molecular computation of solutions to combinatorial problems. Science 266, 1021–1024 (1994).
Phillips, A. & Cardelli, L. A programming language for composable DNA circuits. J. R. Soc. Interface 6, S419–436 (2009).
Qian, L. & Winfree, E. Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201 (2011).
Chatterjee, G., Dalchau, N., Muscat, R. A., Phillips, A. & Seelig, G. A spatially localized architecture for fast and modular DNA computing. Nat. Nanotechnol. 12, 920–927 (2017).
Dalchau, N., Chandran, H., Gopalkrishnan, N., Phillips, A. & Reif, J. Probabilistic analysis of localized DNA hybridization circuits. Acs Synth. Biol. 4, 898–913 (2015).
Wickham, S. F. J. et al. A DNA-based molecular motor that can navigate a network of tracks. Nat. Nanotechnol. 7, 169–173 (2012).
Boemo, M. A., Lucas, A. E., Turberfield, A. J. & Cardelli, L. The formal language and design principles of autonomous DNA walker circuits. ACS Synth. Biol. 5, 878–884 (2016).
Gu, H., Chao, J., Xiao, S. J. & Seeman, N. C. A proximity-based programmable DNA nanoscale assembly line. Nature 465, 202–205 (2010). This study demonstrates a DNA walker that can move on a DNA origami track while collecting proximal cargo molecules on the way, which inspires later studies on DNA machines and robots.
Wang, D. et al. Molecular logic gates on DNA origami nanostructures for microRNA diagnostics. Anal. Chem. 86, 1932–1936 (2014).
Amir, Y. et al. Universal computing by DNA origami robots in a living animal. Nat. Nanotechnol. 9, 353–357 (2014).
Woods, D. et al. Diverse and robust molecular algorithms using reprogrammable DNA self-assembly. Nature 567, 366–372 (2019).
Liu, H., Wang, J., Song, S., Fan, C. & Gothelf, K. V. A DNA-based system for selecting and displaying the combined result of two input variables. Nat. Commun. 6, 10089 (2015).
Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834 (2012). This study reports a device controlled by an aptamer-encoded logic gate, enabling it to expose the payloads conditionally in response to different cues on the cell surface.
DeLuca, M., Shi, Z., Castro, C. E. & Arya, G. Dynamic DNA nanotechnology: toward functional nanoscale devices. Nanoscale Horiz. 5, 182–201 (2020).
Zhang, Y. et al. Dynamic DNA structures. Small 15, e1900228 (2019).
Li, C.-Y. et al. Ionic conductivity, structural deformation, and programmable anisotropy of DNA origami in electric field. ACS Nano 9, 1420–1433 (2015).
Kroener, F., Heerwig, A., Kaiser, W., Mertig, M. & Rant, U. Electrical actuation of a DNA origami nanolever on an electrode. J. Am. Chem. Soc. 139, 16510–16513 (2017).
Kuzyk, A., Yurke, B., Toppari, J. J., Linko, V. & Törmä, P. Dielectrophoretic trapping of DNA origami. Small 4, 447–450 (2008).
Marras, A. E., Zhou, L., Su, H. J. & Castro, C. E. Programmable motion of DNA origami mechanisms. Proc. Natl Acad. Sci. USA 112, 713–718 (2015).
Ramezani, H. & Dietz, H. Building machines with DNA molecules. Nat. Rev. Genet. 21, 5–26 (2020).
Pezzato, C., Cheng, C., Stoddart, J. F. & Astumian, R. D. Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 46, 5491–5507 (2017).
Bazrafshan, A. et al. Tunable DNA origami motors translocate ballistically over μm distances at nm/s speeds. Angew. Chem. Int. Ed. 59, 9514–9521 (2020).
Zhang, Q. et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 8, 6633–6643 (2014).
Jiang, D. et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat. Biomed. Eng. 2, 865–877 (2018).
Jiang, Q. et al. DNA origami as a carrier for circumvention of drug resistance. J. Am. Chem. Soc. 134, 13396–13403 (2012).
Zhao, Y.-X. et al. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano 6, 8684–8691 (2012).
Schüller, V. J. et al. Cellular immunostimulation by CpG-sequence-coated DNA origami structures. ACS Nano 5, 9696–9702 (2011).
Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. https://doi.org/10.1038/s41563-020-0793-6 (2020).
Rahman, M. A. et al. Systemic delivery of Bc12-targeting siRNA by DNA nanoparticles suppresses cancer cell growth. Angew. Chem. Int. Ed. 56, 16023–16027 (2017).
Lee, H. et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 7, 389–393 (2012).
Mei, Q. et al. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 11, 1477–1482 (2011).
Li, J. et al. Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano 5, 8783–8789 (2011).
Liang, L. et al. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 53, 7745–7750 (2014).
Bhatia, D., Surana, S., Chakraborty, S., Koushika, S. P. & Krishnan, Y. A synthetic icosahedral DNA-based host–cargo complex for functional in vivo imaging. Nat. Commun. 2, 339 (2011).
Modi, S. et al. A DNA nanomachine that maps spatial and temporal pH changes inside living cells. Nat. Nanotechnol. 4, 325–330 (2009).
Zhang, H. et al. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. USA 116, 7543–7548 (2019).
Zhang, H. et al. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 15, 3064–3087 (2020).
Wang, P. et al. Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. J. Am. Chem. Soc. 140, 2478–2484 (2018). This study presents a visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells.
Bastings, M. M. C. et al. Modulation of the cellular uptake of DNA origami through control over mass and shape. Nano Lett. 18, 3557–3564 (2018).
Wiraja, C. et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat. Commun. 10, 1147 (2019).
Poon, W., Kingston, B. R., Ouyang, B., Ngo, W. & Chan, W. C. W. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020).
Mikkila, J. et al. Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett. 14, 2196–2200 (2014).
Schaffert, D. H. et al. Intracellular delivery of a planar DNA origami structure by the transferrin-receptor internalization pathway. Small 12, 2634–2640 (2016).
Veneziano, R. et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 15, 716–723 (2020).
Surana, S., Shenoy, A. R. & Krishnan, Y. Designing DNA nanodevices for compatibility with the immune system of higher organisms. Nat. Nanotechnol. 10, 741–747 (2015). This perspective article discusses the immunocompatibility issues of DNA nanotechnology in biomedical applications and proposes possible strategies that could either evade or stimulate the host response.
Engelhardt, F. A. S. et al. Custom-size, functional, and durable DNA origami with design-specific scaffolds. ACS Nano 13, 5015–5027 (2019).
Auvinen, H. et al. Protein coating of DNA nanostructures for enhanced stability and immunocompatibility. Adv. Healthc. Mater. 6, 1700692 (2017).
Steinhauer, C., Jungmann, R., Sobey, T. L., Simmel, F. C. & Tinnefeld, P. DNA origami as a nanoscopic ruler for super-resolution microscopy. Angew. Chem. Int. Ed. 48, 8870–8873 (2009).
Schmied, J. J. et al. DNA origami-based standards for quantitative fluorescence microscopy. Nat. Protoc. 9, 1367–1391 (2014).
Zanacchi, F. C. et al. A DNA origami platform for quantifying protein copy number in super-resolution. Nat. Methods 14, 789–792 (2017).
Jungmann, R. et al. Quantitative super-resolution imaging with qPAINT. Nat. Methods 13, 439–442 (2016).
Kosuri, P., Altheimer, B. D., Dai, M., Yin, P. & Zhuang, X. Rotation tracking of genome-processing enzymes using DNA origami rotors. Nature 572, 136–140 (2019).
Pfitzner, E. et al. Rigid DNA beams for high-resolution single-molecule mechanics. Angew. Chem. Int. Ed. 52, 7766–7771 (2013).
Kilchherr, F. et al. Single-molecule dissection of stacking forces in DNA. Science 353, aaf5508 (2016).
Dutta, P. K. et al. Programmable multivalent DNA origami tension probes for reporting cellular traction forces. Nano Lett. 18, 4803–4811 (2018).
Hariadi, R. F. et al. Mechanical coordination in motor ensembles revealed using engineered artificial myosin filaments. Nat. Nanotechnol. 10, 696–700 (2015).
Iwaki, M., Iwane, A. H., Ikezaki, K. & Yanagida, T. Local heat activation of single myosins based on optical trapping of gold nanoparticles. Nano Lett. 15, 2456–2461 (2015).
Derr, N. D. et al. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science 338, 662–665 (2012).
Endo, M. & Sugiyama, H. Single-molecule imaging of dynamic motions of biomolecules in DNA origami nanostructures using high-speed atomic force microscopy. Acc. Chem. Res. 47, 1645–1653 (2014).
Rajendran, A., Endo, M., Hidaka, K. & Sugiyama, H. Direct and real-time observation of rotary movement of a DNA nanomechanical device. J. Am. Chem. Soc. 135, 1117–1123 (2013).
Endo, M. et al. Direct visualization of the movement of a single T7RNA polymerase and transcription on a DNA nanostructure. Angew. Chem. Int. Ed. 51, 8778–8782 (2012).
Suzuki, Y. et al. DNA origami based visualization system for studying site-specific recombination events. J. Am. Chem. Soc. 136, 211–218 (2014).
Endo, M., Katsuda, Y., Hidaka, K. & Sugiyama, H. Regulation of DNA methylation using different tensions of double strands constructed in a defined DNA nanostructure. J. Am. Chem. Soc. 132, 1592–1597 (2010).
Wickham, S. F. J. et al. Direct observation of stepwise movement of a synthetic molecular transporter. Nat. Nanotechnol. 6, 166–169 (2011).
Funke, J. J. & Dietz, H. Placing molecules with Bohr radius resolution using DNA origami. Nat. Nanotechnol. 11, 47–52 (2016).
Funke, J. J., Ketterer, P., Lieleg, C., Korber, P. & Dietz, H. Exploring nucleosome unwrapping using DNA origami. Nano Lett. 16, 7891–7898 (2016).
Funke, J. J. et al. Uncovering the forces between nucleosomes using DNA origami. Sci. Adv. 2, e1600974 (2016).
Le, J. V. et al. Probing nucleosome stability with a DNA origami nanocaliper. ACS Nano 10, 7073–7084 (2016).
Nickels, P. C. et al. Molecular force spectroscopy with a DNA origami-based nanoscopic force clamp. Science 354, 305–307 (2016).
Xiong, Q. et al. DNA origami post-processing by CRISPR–Cas12a. Angew. Chem. Int. Ed. 59, 3956–3960 (2020).
Kramm, K. et al. DNA origami-based single-molecule force spectroscopy elucidates RNA polymerase III pre-initiation complex stability. Nat. Commun. 11, 2828 (2020).
Martin, T. G. et al. Design of a molecular support for cryo-EM structure determination. Proc. Natl Acad. Sci. USA 113, E7456–E7463 (2016).
Dong, Y. et al. Folding DNA into a lipid-conjugated nanobarrel for controlled reconstitution of membrane proteins. Angew. Chem. Int. Ed. 57, 2072–2076 (2018).
Aksel, T., Yu, Z., Cheng, Y. & Douglas, S. M. Molecular goniometers for single-particle cryo-electron microscopy of DNA-binding proteins. Nat. Biotechnol. https://doi.org/10.1038/s41587-020-0716-8 (2020).
Rinker, S. et al. Self-assembled DNA anostructures for distance-dependent multivalent ligand–protein binding. Nat. Nanotechnol. 3, 418–422 (2008).
Shaw, A. et al. Binding to nanopatterned antigens is dominated by the spatial tolerance of antibodies. Nat. Nanotechnol. 14, 184–190 (2019).
Zhang, P. et al. Capturing transient antibody conformations with DNA origami epitopes. Nat. Commun. 11, 3114 (2020).
Wang, F. et al. Programming PAM antennae for efficient CRISPR–Cas9 DNA editing. Sci. Adv. 6, eaay9948 (2020).
Fisher, P. D. E. et al. A programmable DNA origami platform for organizing intrinsically disordered nucleoporins within nanopore confinement. ACS Nano 12, 1508–1518 (2018).
Ketterer, P. et al. DNA origami scaffold for studying intrinsically disordered proteins of the nuclear pore complex. Nat. Commun. 9, 902 (2018).
Xu, W. et al. A programmable DNA origami platform to organize SNAREs for membrane fusion. J. Am. Chem. Soc. 138, 4439–4447 (2016).
Yang, Y. et al. Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat. Chem. 8, 476–483 (2016).
Zhang, Z., Yang, Y., Pincet, F., Llaguno, M. C. & Lin, C. Placing and shaping liposomes with reconfigurable DNA nanocages. Nat. Chem. 9, 653–659 (2017).
Bian, X., Zhang, Z., Xiong, Q., De Camilli, P. & Lin, C. A programmable DNA origami platform for studying lipid transfer between bilayers. Nat. Chem. Biol. 15, 830–837 (2019).
Czogalla, A. et al. Amphipathic DNA origami nanoparticles to scaffold and deform lipid membrane vesicles. Angew. Chem. Int. Ed. 54, 6501–6505 (2015).
Franquelim, H. G., Khmelinskaia, A., Sobczak, J.-P., Dietz, H. & Schwille, P. Membrane sculpting by curved DNA origami scaffolds. Nat. Commun. 9, 811 (2018).
Grome, M. W., Zhang, Z., Pincet, F. & Lin, C. Vesicle tubulation with self-assembling DNA nanosprings. Angew. Chem. Int. Ed. 57, 5330–5334 (2018).
Grome, M. W., Zhang, Z. & Lin, C. Stiffness and membrane anchor density modulate DNA-nanospring-induced vesicle tubulation. ACS Appl. Mater. Interfaces 11, 22987–22992 (2019).
Journot, C. M. A., Ramakrishna, V., Wallace, M. I. & Turberfield, A. J. Modifying membrane morphology and interactions with DNA origami clathrin-mimic networks. ACS Nano 13, 9973–9979 (2019).
Ghenuche, P., de Torres, J., Moparthi, S. B., Grigoriev, V. & Wenger, J. Nanophotonic enhancement of the Forster resonance energy-transfer rate with single nanoapertures. Nano Lett. 14, 4707–4714 (2014).
Engst, C. R. et al. DNA origami nanopores. Nano Lett. 12, 512–517 (2012).
Wei, R., Martin, T. G., Rant, U. & Dietz, H. DNA origami gatekeepers for solid-state nanopores. Angew. Chem. Int. Ed. 51, 4864–4867 (2012).
Saccà, B. & Niemeyer, C. M. DNA origami: the art of folding DNA. Angew. Chem. Int. Ed. 51, 58–66 (2012).
Xin, Y. et al. Cryopreservation of DNA origami nanostructures. Small 16, 1905959 (2020).
Ponnuswamy, N. et al. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 8, 15654 (2017).
Birac, J. J., Sherman, W. B., Kopatsch, J., Constantinou, P. E. & Seeman, N. C. Architecture with GIDEON, a program for design in structural DNA nanotechnology. J. Mol. Graph. Model. 25, 470–480 (2006).
Ke, Y. et al. Multilayer DNA origami packed on a square lattice. J. Am. Chem. Soc. 131, 15903–15908 (2009).
Williams, S. et al. in DNA Computing Vol. 5347 (eds Goel, A., Simmel, F. C. & Sosik, P.) 90–101 (Springer, 2009).
Kim, D.-N., Kilchherr, F., Dietz, H. & Bathe, M. Quantitative prediction of 3D solution shape and flexibility of nucleic acid nanostructures. Nucleic Acids Res. 40, 2862–2868 (2011).
Ouldridge, T. E., Louis, A. A. & Doye, J. P. K. Structural, mechanical, and thermodynamic properties of a coarse-grained DNA model. J. Chem. Phys. 134, 085101 (2011).
Sharma, R., Schreck, J. S., Romano, F., Louis, A. A. & Doye, J. P. K. Characterizing the motion of jointed DNA nanostructures using a coarse-grained model. ACS Nano 11, 12426–12435 (2017).
Shi, Z., Castro, C. E. & Arya, G. Conformational dynamics of mechanically compliant DNA nanostructures from coarse-grained molecular dynamics simulations. ACS Nano 11, 4617–4630 (2017).
Huang, C.-M., Kucinic, A., Le, J. V., Castro, C. E. & Su, H.-J. Uncertainty quantification of a DNA origami mechanism using a coarse-grained model and kinematic variance analysis. Nanoscale 11, 1647–1660 (2019).
Stephanopoulos, N. Peptide–oligonucleotide hybrid molecules for bioactive nanomaterials. Bioconjugate Chem. 30, 1915–1922 (2019).
Yang, Y. R., Liu, Y. & Yan, H. DNA nanostructures as programmable biomolecular scaffolds. Bioconjugate Chem. 26, 1381–1395 (2015). This review focuses on using DNA nanostructures to precisely programme the spatial arrangements of biomolecules, especially proteins, which is fundamental in applications including catalysis, drug delivery, bioimaging and biophysics.
Marras, A. E., Zhou, L., Kolliopoulos, V., Su, H. J. & Castro, C. E. Directing folding pathways for multi-component DNA origami nanostructures with complex topology. N. J. Phys. 18, 055005 (2016).
Myhrvold, C. et al. Barcode extension for analysis and reconstruction of structures. Nat. Commun. 8, 14698 (2017).
Iinuma, R. et al. Polyhedra self-assembled from DNA tripods and characterized with 3D DNA-PAINT. Science 344, 65–69 (2014).
Zhao, Z., Yan, H. & Liu, Y. A route to scale up DNA origami using DNA tiles as folding staples. Angew. Chem. Int. Ed. 49, 1414–1417 (2010).
Zhao, Z., Liu, Y. & Yan, H. Organizing DNA origami tiles into larger structures using preformed scaffold frames. Nano Lett. 11, 2997–3002 (2011).
Woo, S. & Rothemund, P. W. K. Self-assembly of two-dimensional DNA origami lattices using cation-controlled surface diffusion. Nat. Commun. 5, 4889 (2014).
Ducani, C., Kaul, C., Moche, M., Shih, W. M. & Högberg, B. Enzymatic production of ‘monoclonal stoichiometric’ single-stranded DNA oligonucleotides. Nat. Methods 10, 647–652 (2013).
Schmidt, T. L. et al. Scalable amplification of strand subsets from chip-synthesized oligonucleotide libraries. Nat. Commun. 6, 8634 (2015).
Praetorius, F. et al. Biotechnological mass production of DNA origami. Nature 552, 84–87 (2017).
Gerling, T., Kube, M., Kick, B. & Dietz, H. Sequence-programmable covalent bonding of designed DNA assemblies. Sci. Adv. 4, eaau1157 (2018).
Cassinelli, V. et al. One-step formation of “chain-armor”-stabilized DNA nanostructures. Angew. Chem. Int. Ed. 54, 7795–7798 (2015).
Chopra, A., Krishnan, S. & Simmel, F. C. Electrotransfection of polyamine folded DNA origami structures. Nano Lett. 16, 6683–6690 (2016).
Ahmadi, Y., De Llano, E. & Barišić, I. (Poly)Cation-induced protection of conventional and wireframe DNA origami nanostructures. Nanoscale 10, 7494–7504 (2018).
Wang, S.-T. et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc. Natl Acad. Sci. USA 117, 6339–6348 (2020).
Perkel, J. M. The race for enzymatic DNA synthesis heats up. Nature 566, 565 (2019).
He, Y. & Liu, D. R. Autonomous multistep organic synthesis in a single isothermal solution mediated by a DNA walker. Nat. Nanotechnol. 5, 778–782 (2010).
Meng, W. et al. An autonomous molecular assembler for programmable chemical synthesis. Nat. Chem. 8, 542–548 (2016).
Lauback, S. et al. Real-time magnetic actuation of DNA nanodevices via modular integration with stiff micro-levers. Nat. Commun. 9, 1446 (2018).
Murugan, A., Zeravcic, Z., Brenner, M. P. & Leibler, S. Multifarious assembly mixtures: systems allowing retrieval of diverse stored structures. Proc. Natl Acad. Sci. USA 112, 54–59 (2015).
He, X. et al. Exponential growth and selection in self-replicating materials from DNA origami rafts. Nat. Mater. 16, 993–997 (2017).
Pinheiro, V. B. et al. Synthetic genetic polymers capable of heredity and evolution. Science 336, 341–344 (2012).
Hoshika, S. et al. Hachimoji DNA and RNA: a genetic system with eight building blocks. Science 363, 884–887 (2019).
Geary, C., Rothemund, P. W. & Andersen, E. S. RNA nanostructures. A single-stranded architecture for cotranscriptional folding of RNA nanostructures. Science 345, 799–804 (2014).
Zhang, Y. et al. A semi-synthetic organism that stores and retrieves increased genetic information. Nature 551, 644–647 (2017).
Matthies, M. et al. Triangulated wireframe structures assembled using single-stranded DNA tiles. ACS Nano 13, 1839–1848 (2019).
Dai, M., Jungmann, R. & Yin, P. Optical imaging of individual biomolecules in densely packed clusters. Nat. Nanotechnol. 11, 798–807 (2016).
Praetorius, F. & Dietz, H. Self-assembly of genetically encoded DNA–protein hybrid nanoscale shapes. Science 355, eaam5488 (2017).
Li, M. et al. In vivo production of RNA nanostructures via programmed folding of single-stranded RNAs. Nat. Commun. 9, 2196 (2018).
Voigt, N. V. et al. Single-molecule chemical reactions on DNA origami. Nat. Nanotechnol. 5, 200–203 (2010).
Langecker, M. et al. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 338, 932–936 (2012).
Knudsen, J. B. et al. Routing of individual polymers in designed patterns. Nat. Nanotechnol. 10, 892–898 (2015).
Li, N. et al. Precise organization of metal and metal oxide nanoclusters into arbitrary patterns on DNA origami. J. Am. Chem. Soc. 141, 17968–17972 (2019).
Aghebat Rafat, A., Sagredo, S., Thalhammer, M. & Simmel, F. C. Barcoded DNA origami structures for multiplexed optimization and enrichment of DNA-based protein-binding cavities. Nat. Chem. 12, 852–859 (2020).
Rosier, B. J. H. M. et al. Proximity-induced caspase-9 activation on a DNA origami-based synthetic apoptosome. Nat. Catal. 3, 295–306 (2020).
Acknowledgements
C.F. and J.L. were supported by the National Natural Science Foundation of China (21991134, 21834007) and the Shanghai Municipal Science and Technology Commission (19JC1410300). K.V.G. and M.A.D.N. were supported by DNA-Based Modular Nanorobotics (DNA-Robotics) and the Marie Curie Innovative Training Network (MRC ITN) under EU H2020 (Project ID: 765703). P.Z. and N.L. were supported by a European Research Council (ERC Dynamic Nano) grant. B.S. was supported by the Deutsche Forschungsgemeinschaft (CRC-1093).
Author information
Authors and Affiliations
Contributions
Introduction (C.F. and J.L.); Experimentation (S.D., N.L., H.Y. and P.Z.); Results (B.S.); Applications (C.F., J.L., C.L., L.L., K.V.G. and M.A.D.N.); Reproducibility and data deposition (C.F., N.L. and P.Z.); Limitations and optimizations (C.L. and L.L.); Outlook (F.C.S.); Overview of the Primer (C.F.).
Corresponding authors
Ethics declarations
Competing interests
C.F. declares an issued Chinese patent (patent number 2018112787266) and a Chinese patent application (2016111794282) based on technologies described in this Primer. F.C.S. has patent applications on DNA origami membrane channels (EP2695949B1) and the electrically driven DNA robotic arm (EP3607646A1). All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Methods Primers thanks A. R. Chandrasekaran, S. Lee, S. Pecic, R. Shetty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Adenita: https://www.samson-connect.net/element/dda2a078-1ab6-96ba-0d14-ee1717632d7a.html
CaDNAno: https://cadnano.org/
CanDo: http://cando-DNA-origami.org/
COSM: http://vsb.fbb.msu.ru/cosm
DAEDALUS: http://daedalus-DNA-origami.org/
EMAN2: https://blake.bcm.edu/emanwiki/EMAN2
GenBank: https://www.ncbi.nlm.nih.gov/genbank
METIS: http://metis-DNA-origami.org/
Molecular programming: http://molecular-programming.org/
oxDNA: https://oxdna.org/
oxView: https://sulcgroup.github.io/oxdna-viewer/
PERDIX: http://perdix-DNA-origami.org/
TALOS: http://talos-DNA-origami.org/
Tiamat: http://yanlab.asu.edu/Resources.html
vHelix: http://vhelix.net/
Glossary
- Holliday junction
-
A four-stranded cross-shaped DNA structure (named after British geneticist Robin Holliday) that forms during the process of genetic recombination.
- DNA nanotechnology
-
A branch of nanotechnology concerned with the design, study and application of DNA-based synthetic structures to take advantage of the physical and chemical properties of DNA.
- DNA tiles
-
DNA structures that as building blocks can be tiled into higher order (usually periodic) structures.
- DNA origami
-
A class of technologies for building DNA nanostructures by folding a long single-stranded DNA (scaffold) into desired shapes via base pairing.
- Scaffold
-
A long single-stranded DNA serving as the major component of a DNA origami structure, which will be folded into a defined shape.
- Staples
-
Short single-stranded DNAs that help fold the scaffold DNA via crossover base pairing.
- Addressable points
-
The locations of staple DNAs, including their extensions or modifications, on a DNA origami structure. These points can be prescribed as each staple has a globally unique base sequence (a unique address).
- Base stacking
-
A stacking arrangement of the planes of nucleobases or base pairs in the structure of nucleic acids, leading to a strong π–π interaction vertical to the planes, which is a major force that stabilizes DNA duplex structures.
- Enzyme cascades
-
Groups of enzymes in which the reaction product of one enzyme is the substrate for the next.
- Abstraction
-
The translation of concrete DNA reactions into abstracted algorithms and instructions. By this method, the complex details are hidden from the persons operating the computing systems.
- In vivo computation
-
Molecular computation implemented in living organisms, whose inputs/outputs are often interfaced with biological pathways/functions.
- DNA origami robot
-
A molecular machine made by DNA origami that can autonomously perform specified task(s) with precise motions at the nanoscale.
- Avidity
-
The molecular binding strength as a result of multiple, non-covalent interactions, for example between an antibody and a complex antigen.
- Tectons
-
Structural motifs that serve as units for assembly of higher order structures.
- Xeno-nucleic acids
-
(XNAs). Artificially synthesized nucleic acids that do not exist in nature (for example, nucleic acids carrying unnatural backbones, bases or chemical modifications).
Rights and permissions
About this article
Cite this article
Dey, S., Fan, C., Gothelf, K.V. et al. DNA origami. Nat Rev Methods Primers 1, 13 (2021). https://doi.org/10.1038/s43586-020-00009-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-020-00009-8
This article is cited by
-
Universal, label-free, single-molecule visualization of DNA origami nanodevices across biological samples using origamiFISH
Nature Nanotechnology (2024)
-
Imaging DNA origami by fluorescence in situ hybridization
Nature Nanotechnology (2024)
-
Artificial cells for in vivo biomedical applications through red blood cell biomimicry
Nature Communications (2024)
-
Progress and prospects of chiral nanomaterials for biosensing platforms
Rare Metals (2024)
-
Biodistribution and function of coupled polymer-DNA origami nanostructures
Scientific Reports (2023)