NATIONAL MUSEUM OF NATURAL HISTORY
Scientists Find Blue-Green Algae Chemical with Cancer Fighting Potential
The discovery shows how studying marine biodiversity can enhance biomedical research.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/blogging/featured/Red_coral_under_water_with_green_hair-like_structures_on_it.jpg)
Blue-green algae, or cyanobacteria, are some of the oldest organisms on Earth, appearing in the fossil record over 3.5 billion years ago. But there is more to these photosynthetic bacteria than their long history. One species produces a chemical compound that shows potential for further research as a novel chemotherapy drug.
New research in the journal Proceedings of the National Academy of Sciences explains how the compound, gatorbulin-1 (GB1), from a cyanobacteria species in south Florida, may have significant anti-cancer activity. This discovery by scientists at the Smithsonian’s National Museum of Natural History and University of Florida (UF) shows how studying marine biodiversity can enhance biomedical research. Gatorbulin-1’s name pays tribute to the UF researchers and global partners who led the way to its discovery and characterization.
“The ocean is relatively unexplored. It is where most of our biological and chemical diversity is undiscovered,” said Dr. Hendrik Luesch, a medicinal chemist, director of the Center for Natural Products, Drug Discovery and Development at the University of Florida and the lead author of the new paper. “We’re interested in locations with high marine biodiversity, because that means there are many organisms communicating and fighting, using bioactive compounds that we can pivot for drug development.”
From defense to drugs
Cyanobacteria are single-celled organisms that live on land and in water all around the world. But even these simple creatures have complex relationships with the world around them.
Cyanobacteria don’t have claws, teeth or a threatening growl for defense. Instead, they use chemicals to protect themselves from predators. Their chemicals also help the bacteria communicate.
“We have studied a series of compounds called quorum sensing inhibitors that effect the chemical cues that bacteria use to communicate,” said Dr. Valerie Paul, a chemical ecologist and head scientist at the Smithsonian Marine Station. Quorum sensing is the name for how bacteria communicate using chemical signals.
Paul and Luesch examine cyanobacteria’s defense and communication compounds to test for biomedical properties. Often, they realize the compound’s medicinal potential before they understand why cyanobacteria use it.
In the new study, gatorbulin-1 is shown to have significant anti-cancer activity with potential to be developed into a new drug. Luesch and Paul understand how GB1 could be important to humans but it is less clear how the cyanobacterium uses it.
“Nature has already optimized these compounds and, in some cases, we don’t know what for,” Paul said. “My strong feeling as a chemical ecologist is that they are being made for a purpose. Gatorbulin-1 wasn’t made to be a potential anti-cancer drug or target humans but it’s toxicity to cells is serving some purpose in the cyanobacterium naturally.”
The path from ocean to laboratory
The blue-green algae species that is tentatively identified as Lyngbya confervoides was discovered over a decade ago when Paul first began collecting the species. She quickly saw that it was producing many different compounds, so she sent samples to her collaborator, Luesch, for further study.
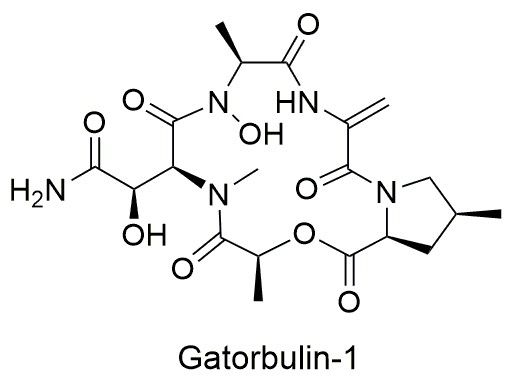
But finding a new compound, like GB1, and learning enough about it to confidently say that it has the potential to be a new drug can be a lengthy process — which doesn’t include the additional time and testing it then takes to turn the compound into a safe, approved and functional drug.
The first part of the process is compound isolation and demonstrating that the purified compound can selectively kill cancer cells. Prompted by this finding, Luesch’s team worked to figure out how to synthesize the compound in the lab. Having a reliable way to produce GB1 is important in being able to conduct in-depth studies.
“We usually can’t go out and constantly collect more of the cyanobacteria,” Luesch said. “It’s fun diving and snorkeling but, at the end of the day, you’re lucky if you find enough of the organism again to isolate enough material for advanced studies. As organic chemists, we can recreate these natural molecules in larger quantities in the laboratory without relying on the cyanobacteria.”
GB1’s novelty added additional steps to the synthesis process. “There are so many ways to put a molecule together and you don’t necessarily know upfront what is the best way,” Luesch said.
Next, Luesch’s team tested the compound against numerous distinct cancer cells to figure out how GB1 worked. The team found that GB1 targets a protein in cells called tubulin, which is the protein that cells require during cell division and use to build their inner scaffolding. While there are already chemotherapy drugs that target tubulin, Luesch and collaborators in Spain showed that GB1 is special because it interacts with tubulin in a new way.
Now Luesch, Paul and their team are eager to see if GB1 has real-world potential to become a cancer-fighting medicine.
“Ultimately, we need additional pharmacological, toxicological and efficacy studies to see how gatorbulin-1 will perform compared to other compounds,” Luesch said.
Healing abilities of biochemical warfare

Organic chemists often turn towards nature for drug discovery research. For example, compounds from land organisms like plants and fungi have led to important drugs like penicillin that are now staples for modern medicine. But the ocean, which makes up almost three quarters of the Earth’s surface, remains largely unexplored.
“We have entire groups of organisms in the ocean that don’t exist on land and have undergone completely different evolutionary pressures over time,” Paul said.
Just like cyanobacteria, many other marine species lack physical defenses and have evolved chemicals for defense and communication.
“It’s really chemical warfare out in the oceans” Luesch said. “The more warfare or communication that is out there, the better for us because it means more active compounds that we can try to put to good use for humankind.”
All of those evolved and under-studied compounds could hold the starting points for researchers seeking to develop new drugs.
“From a chemist’s point of view, even though I am a chemical ecologist, this biodiversity equates to chemical diversity,” Paul said. “You can find a whole suite of things in the ocean that we never even dreamed of.”
Related Stories:
Scientists Describe New Species of Rare Bryde’s Whale
Shocking Study Finds Electric Eels Hunt Together
How Scientists Learn What Lives in the Deep Ocean
Rare Megamouth Shark Arrives at the Smithsonian

