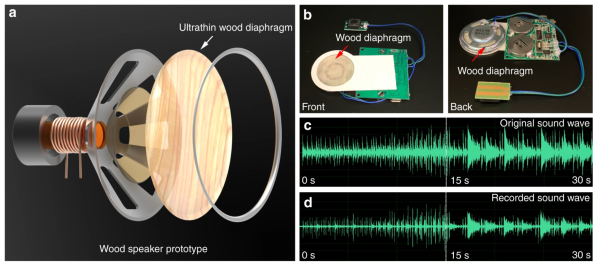
Researchers have created an audio speaker using ultra-thin wood film. The new material demonstrates high tensile strength and increased Young’s modulus, as well as acoustic properties contributing to higher resonance frequency and greater displacement amplitude compared to a commercial polypropylene diaphragm in an audio speaker.
Typically, acoustic membranes have to remain very thin (on the micron scale) and robust in order to allow for a highly sensitive frequency response and vibrational amplitude. Materials made from plastic, metal, ceramic, and carbon have been used by engineers and physicists in an attempt to enhance the quality of sound. While plastic thin films are most commonly manufactured, they have a pretty bad impact on the environment. Meanwhile, metal, ceramic, and carbon-based materials are more expensive and less attractive to manufacturers as a result.
Cellulose-based materials have been making an entrance in acoustics research with their environmentally friendly nature and natural wooden structure. Materials like bagasse, wood fibers, chitin, cotton, bacterial cellulose, and lignocellulose are all contenders for effective alternatives to parts currently produced from plastics.
The process for building the ultra-thin film involved removing lignin and hemicellulose from balsa wood, resulting in a highly porous material. The result is hot pressed for a thickness reduction of 97%. The cellulose nano-fibers remain oriented but more densely packed compared to natural wood. In addition, the fibers required higher energy to be pulled apart while remaining flexible and foldable.
At one point in time, plastics seemed to be the hottest new material, but perhaps wood is making a comeback?
[Thanks Qes for the tip!]