Abstract
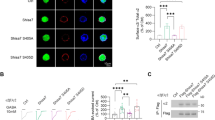
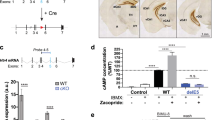
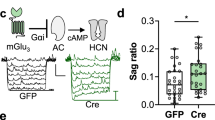
ATP signaling and surface P2X4 receptors are upregulated selectively in neurons and/or glia in various CNS disorders including anxiety, chronic pain, epilepsy, ischemia, and neurodegenerative diseases. However, the cell-specific functions of P2X4 in pathological contexts remain elusive. To elucidate P2X4 functions, we created a conditional transgenic knock-in P2X4 mouse line (Floxed P2X4mCherryIN) allowing the Cre activity-dependent genetic swapping of the internalization motif of P2X4 by the fluorescent mCherry protein to prevent constitutive endocytosis of P2X4. By combining molecular, cellular, electrophysiological, and behavioral approaches, we characterized two distinct knock-in mouse lines expressing noninternalized P2X4mCherryIN either exclusively in excitatory forebrain neurons or in all cells natively expressing P2X4. The genetic substitution of wild-type P2X4 by noninternalized P2X4mCherryIN in both knock-in mouse models did not alter the sparse distribution and subcellular localization of P2X4 but increased the number of P2X4 receptors at the surface of the targeted cells mimicking the pathological increased surface P2X4 state. Increased surface P2X4 density in the hippocampus of knock-in mice altered LTP and LTD plasticity phenomena at CA1 synapses without affecting basal excitatory transmission. Moreover, these cellular events translated into anxiolytic effects and deficits in spatial memory. Our results show that increased surface density of neuronal P2X4 contributes to synaptic deficits and alterations in anxiety and memory functions consistent with the implication of P2X4 in neuropsychiatric and neurodegenerative disorders. Furthermore, these conditional P2X4mCherryIN knock-in mice will allow exploring the cell-specific roles of P2X4 in various physiological and pathological contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69.
Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–95.
Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–53.
Kaczmarek-Hajek K, Zhang J, Kopp R, Grosche A, Rissiek B, Saul A, et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. eLife. 2018;7:e36217.
Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–5.
Mori M, Heuss C, Gahwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol. 2001;535:115–23.
Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–86.
Pougnet JT, Toulme E, Martinez A, Choquet D, Hosy E, Boue-Grabot E. ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron. 2014;83:417–30.
Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014;12:e1001747.
Boué-Grabot E, Pankratov Y. Modulation of central synapses by astrocyte-released ATP and postsynaptic P2X receptors. Neural Plast. 2017;2017:9454275.
Illes P, Verkhratsky A. Purinergic neurone-glia signalling in cognitive-related pathologies. Neuropharmacology. 2016;104:62–75.
Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–8.
Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–20.
Suurväli J, Boudinot P, Kanellopoulos J, Rüütel Boudinot S. P2X4: a fast and sensitive purinergic receptor. Biomed J. 2017;40:245–56.
Xu J, Bernstein AM, Wong A, Lu XH, Khoja S, Yang XW, et al. P2X4 receptor reporter mice: sparse brain expression and feeding-related presynaptic facilitation in the arcuate nucleus. J Neurosci. 2016;36:8902–20.
Yeung D, Kharidia R, Brown SC, Gorecki DC. Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol Dis. 2004;15:212–20.
Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9.
Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A P2X receptors and synaptic plasticity. Neuroscience. 2009;158:137–148.
Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D'Ambrosi N, et al. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98.
Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharm Ther. 2006;109:297–324.
Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Disco. 2008;7:575–90.
Apolloni S, Montilli C, Finocchi P, Amadio S. Membrane compartments and purinergic signalling: P2X receptors in neurodegenerative and neuroinflammatory events. FEBS J. 2009;276:354–64.
Volonte C, Apolloni S, Parisi C, Amadio S. Purinergic contribution to amyotrophic lateral sclerosis. Neuropharmacology. 2016;104:180–93.
Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–73.
Varma R, Chai Y, Troncoso J, Gu J, Xing H, Stojilkovic SS, et al. Amyloid-beta induces a caspase-mediated cleavage of P2X4 to promote purinotoxicity. Neuromolecular Med. 2009;11:63–75.
Casanovas A, Hernandez S, Tarabal O, Rossello J, Esquerda JE. Strong P2X4 purinergic receptor-like immunoreactivity is selectively associated with degenerating neurons in transgenic rodent models of amyotrophic lateral sclerosis. J Comp Neurol. 2008;506:75–92.
Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–24.
Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–85.
Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–49.
Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen DJ, Murrell-Lagnado RD. Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci. 2005;118:3073–80.
Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–53.
Cao Q, Zhong XZ, Zou Y, Murrell-Lagnado R, Zhu MX, Dong XP. Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J Cell Biol. 2015;209:879–94.
Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, et al. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem. 2014;289:17658–67.
Robinson LE, Murrell-Lagnado RD. The trafficking and targeting of P2X receptors. Front Cell Neurosci. 2013;7:233.
Andries M, Van Damme P, Robberecht W, Van Den Bosch L. Ivermectin inhibits AMPA receptor-mediated excitotoxicity in cultured motor neurons and extends the life span of a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;25:8–16.
Khoja S, Huynh N, Asatryan L, Jakowec MW, Davies DL. Reduced expression of purinergic P2X4 receptors increases voluntary ethanol intake in C57BL/6J mice. Alcohol. 2018;68:63–70.
Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, et al. Contribution of P2X4 receptors to ethanol intake in male C57BL/6 mice. Neurochem Res. 2014;39:1127–39.
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83.
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–8.
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21.
Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–300.
Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem. 2011;286:19993–20004.
Toulme E, Soto F, Garret M, Boue-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharm. 2006;69:576–87.
Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugene E, et al. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J Neurosci. 2013;33:15488–503.
Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010.
Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, et al. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 2009;29:4829–35.
Bertin E, Martinez A. Boue-Grabot E. P2X Electrophysiology and Surface Trafficking in Xenopus Oocytes. Methods Mol Biol. 2020;2041:243–59.
Belzung C. Hippocampal mossy fibres: implication in novelty reactions or in anxiety behaviours? Behav Brain Res. 1992;51:149–55.
Renner MJ, Bennett AJ, White JC. Age and sex as factors influencing spontaneous exploration and object investigation by preadult rats (Rattus norvegicus). J Comp Psychol. 1992;106:217–27.
Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharm Biochem Behav. 1986;24:525–9.
Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem. 2000;73:31–48.
Bergmann P, Garcia de Paco A, Rissiek B, Menzel S, Dubberke G, Hua J et al. Generation and characterization of specific monoclonal antibodies and Nanobodies directed against the ATP-gated channel P2X4. Front Cell Neurosci. 2019;13:498.
Lê KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–90.
Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62.
Ulmann L, Levavasseur F, Avignone E, Peyroutou R, Hirbec H, Audinat E, et al. Involvement of P2X4 receptors in hippocampal microglial activation after status epilepticus. Glia. 2013;61:1306–19.
Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–6.
Nicole O, Hadzibegovic S, Gajda J, Bontempi B, Bem T, Meyrand P. Soluble amyloid beta oligomers block the learning-induced increase in hippocampal sharp wave-ripple rate and impair spatial memory formation. Sci Rep. 2016;6:22728.
Layhadi JA, Turner J, Crossman D, Fountain SJ. ATP evokes Ca(2+) responses and CXCL5 secretion via P2X4 receptor activation in human monocyte-derived macrophages. J Immunol. 2018;200:1159–68.
Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26.
Stokes L, Layhadi JA, Bibic L, Dhuna K, Fountain SJ. P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Front Pharm. 2017;8:291.
Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL. P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front Neurosci. 2014;8:176.
Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–17.
Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34:213–20.
Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–9.
Jo YH, Boue-Grabot E. Interplay between ionotropic receptors modulates inhibitory synaptic strength. Commun Integr Biol. 2011;4:706–9.
Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, Bortolato M et al. Sociocommunicative and sensorimotor impairments in male P2X4-deficient mice. Neuropsychopharmacology. 2013;38:1993–2002.
Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–83.
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609.
Hafner S, Wagner K, Weber S, Groger M, Wepler M, McCook O, et al. Role of the purinergic receptor P2XR4 after blunt chest trauma in cigarette smoke-exposed mice. Shock. 2017;47:193–9.
Chen H, Xia Q, Feng X, Cao F, Yu H, Song Y, et al. Effect of P2X4R on airway inflammation and airway remodeling in allergic airway challenge in mice. Mol Med Rep. 2016;13:697–704.
Yang T, Shen JB, Yang R, Redden J, Dodge-Kafka K, Grady J, et al. Novel protective role of endogenous cardiac myocyte P2X4 receptors in heart failure. Circ Heart Fail. 2014;7:510–8.
Pettengill MA, Marques-da-Silva C, Avila ML, d'Arc dos Santos Oliveira S, Lam VW, Ollawa I, et al. Reversible inhibition of Chlamydia trachomatis infection in epithelial cells due to stimulation of P2X(4) receptors. Infect Immun. 2012;80:4232–8.
Gonzales E, Julien B, Serriere-Lanneau V, Nicou A, Doignon I, Lagoudakis L, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52:54–62.
Acknowledgements
We thank G. Dabee for the production of all transgenic mice at the animal facility, H. Orignac for help with Xenopus facilities and E. Normand for stereotaxic injection. We thank the Mouse Clinical Institute (Institut Clinique de la Souris, MCI/ICS) in the Genetic Engineering and Model Validation Department who established the mouse mutant floxed P2X4mCherryIN line. We are grateful to F. Rassendren (IGF, Montpellier) for providing P2X4KO mice. We also thank the biochemistry facility of Bordeaux Neurocampus. Electron microscopy was performed at the Bordeaux Imaging Center, a service unit of the CNRS-INSERM and Bordeaux University. This work was supported by CNRS, University of Bordeaux, a grant LabEx BRAIN ANR-10-LABX-43 to EB-G and EB, a grant from Inserm for the generation of the mouse line to EB-G, the Louise and Alan Edwards Foundation, an awarded grant from Quebec Pain Research Network (QPRN) to TD, International Ph. D program of the IdEx of Bordeaux to EB-G and PS and DFG grant SFB1328-Z02 to FK-N.
Author information
Authors and Affiliations
Contributions
EB, TD, KSP, AM, J-TP, ED, A-EA, ET, MR, FG, ON performed the experiments and analyzed the data. PS, BB, SL, FG, SB, ON and EB-G designed the experiments and analyzed the data. PB, EB, FK-N contributed with key reagents. EB-G conceived the knock-in mice and the study. KSP, ON, and EB-G wrote the paper. All authors commented the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All experimental procedures complied with official European guidelines for the care and use of laboratory animals (Directive 2010/63/UE).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bertin, E., Deluc, T., Pilch, K.S. et al. Increased surface P2X4 receptor regulates anxiety and memory in P2X4 internalization-defective knock-in mice. Mol Psychiatry 26, 629–644 (2021). https://doi.org/10.1038/s41380-019-0641-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0641-8
This article is cited by
-
Elucidating the mechanisms underlying astrocyte-microglia crosstalk in hippocampal neuroinflammation induced by acute diquat exposure
Environmental Science and Pollution Research (2024)
-
Increased ATP Release and Higher Impact of Adenosine A2A Receptors on Corticostriatal Plasticity in a Rat Model of Presymptomatic Parkinson’s Disease
Molecular Neurobiology (2023)
-
Increased surface P2X4 receptors by mutant SOD1 proteins contribute to ALS pathogenesis in SOD1-G93A mice
Cellular and Molecular Life Sciences (2022)
-
The Purinergic System as a Target for the Development of Treatments for Bipolar Disorder
CNS Drugs (2022)
-
Contribution of P2X4 receptor in pain associated with rheumatoid arthritis: a review
Purinergic Signalling (2021)