Abstract
Molecules involved in developmental signaling pathways have emerged as therapeutic targets for various rheumatic diseases. New research sheds light on the consequences of interfering with these processes.
Introduction
Improvements in the control of chronic inflammation achieved by selectively targeting specific cytokines or immune-cell types have not only improved patient outcomes in arthritic diseases such as rheumatoid arthritis, ankylosing spondylitis (AS) and psoriatic arthritis, but also shifted the attention of the research community toward the processes involved in skeletal damage and repair. Molecular signaling pathways that have critical roles in embryonic development, such as those involving Wnts and their antagonists, seem to be involved in tissue repair and remodeling in adult life and disease. Uderhardt et al.[1] have recently published results showing that blockade of Dickkopf-1 (DKK1), a Wnt receptor antagonist, induces fusion of the sacroiliac joints in a tumor necrosis factor (TNF)-driven arthritis model. Manipulation of developmental pathways might, however, also be associated with cell transformation and cancer (Box 1). The biology of Wnts and their antagonists is a typical example of this conundrum. Research by Kansara et al.[2] shows that Wnt inhibitory factor 1 (WIF1), an endogenously secreted Wnt pathway antagonist, is epigenetically silenced in human osteosarcoma, and that targeted deletion of Wif1 accelerates the formation of radiation-induced osteosarcomas in mice.
The formation of new cartilage and bone leading to spine or joint ankylosis is a major determinant of long-term outcome in AS and related spondyloarthritides. The relationship between chronic inflammation in the spine and joints and ankylosis remains controversial, as successful treatment of inflammation does not seem to affect the radiographic progression of the disease.[3] The molecular mechanisms underlying ankylosis also remain unclear. The components of pathways involved in cartilage and bone formation during development, such as Wnts and bone morphogenetic proteins, have been proposed as key players in these processes (Figure 1). Our group has demonstrated that inhibition of bone morphogenetic proteins can prevent peripheral joint ankylosis in mice.[4] Also, inhibition of DKK1 transforms the destructive phenotype of some mouse models of arthritis into a remodeling phenotype in the peripheral joints.[5] Until now, however, with the exception of a meeting abstract,[6] no published data were available on the molecular signals that lead to spine and sacroiliac joint ankylosis, which are, much more so than peripheral joint problems, the hallmarks of AS.
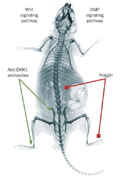
Figure 1.
Manipulating signaling pathways influences ankylosis in mice. Treatment with antibodies against DKK1, a Wnt antagonist, stimulates ankylosis (green arrows) in peripheral[5] and sacroiliac[1] joints. Overexpression of noggin, a BMP antagonist, inhibits ankylosis (red arrows) in the peripheral joints[4] and spine.[6] Abbreviations: BMP, bone morphogenetic protein; DKK1, Dickkopf1.
In the paper mentioned above, Uderhardt et al.[1] show that blocking DKK1 affects the outcome of arthritis in not only peripheral but also sacroiliac joints. Human TNF transgenic mice develop arthritis of the sacroiliac joints, leading to severe erosive changes and loss of the lining cartilage. Blocking TNF in these mice reduced inflammation but did not lead to ankylosis. By contrast, blocking DKK1 did not affect inflammation but led to cartilage and bone formation in inflamed joints. This report is the first to suggest a cellular and molecular mechanism underlying sacroiliac joint ankylosis. However, before these findings can be translated into clinical practice, a number of important points remain to be addressed.
First, the human TNF transgenic mouse model does not provide an opportunity to study spinal ankylosis, which, to a much greater extent than bony bridging of the sacroiliac joints, contributes to the functional outcomes of the disease. Sacroiliac joints -- despite the fact that they are part of the axial skeleton -- show extensive structural similarities to peripheral joints, in particular the presence of a synovium. In TNF transgenic mice, destructive synovitis is the dominant feature. The microscopic images provided in the Uderhardt et al.[1] paper suggest that joint ankylosis occurs after destruction of the articular cartilage, and probably also the fibrocartilage of the sacroiliac joint. The nature of this bone formation is more difficult to assess, and it seems, as the authors point out, that at least three different biological processes could be implicated: endochondral bone formation, membranous bone formation and cartilage metaplasia.
Second, as the paper by Kansara et al.[2] shows, derepression of Wnts -- that is, by silencing Wnt inhibitors -- might accelerate or increase the occurrence of rare tumors such as osteosarcoma, particularly in young individuals. A number of reports have been published on the role of Wnt signaling in rheumatic diseases (reviewed elsewhere[7,8]), and targeting the Wnt pathway seems an exciting option for influencing tissue responses in diseases such as chronic arthritis. The obvious challenge in this concept will be to appropriately control Wnt signaling.
Third, the development of drugs and therapeutic strategies that influence tissue responses in joint diseases may differ from those that aim to control inflammation and tissue destruction. Goals and outcome parameters need to be carefully defined. Rather than completely blocking a given pathway, altering agonist-antagonist balances seems an approach more likely to support repair and restore homeostasis. Examples of such rebalancing strategies can be found in the use of bone anabolic treatments for osteoporosis.[9] Administration of parathyroid hormone (PTH) is a strongly anabolic treatment that is effective in patients in whom antiresorptive strategies alone have failed. The use of PTH is generally considered safe, although experience is still limited. This favorable safety profile might be influenced by the fact that PTH is usually administered intermittently in middle-aged and older individuals and hence exposure is not continuous. In terms of oncogenesis, it is important to realize that the findings reported by Kansara et al.[2] highlight some spontaneous development of osteosarcomas, but that the effect of WIF1 deletion was strongly enhanced by radiation. By contrast, different knockout models for other Wnt antagonists have not reportedly been associated with spontaneous tumor formation.
Finally, pathways such as Wnt signaling are very complex and involve a number of different ligands, receptors and endogenous antagonists and the activation of distinct intracellular signaling pathways. Many components of the Wnt pathway are theoretically targets for new drug discovery. Selecting a tissue-appropriate target may be an important consideration in order to limit toxicity. Targeting this pathway implies interference with a cellular compartment quite different from that which drives the autoimmune reactions that characterize immune-mediated inflammatory diseases. Targeting Wnts and related signaling pathways affects distinct tissue-resident cell populations, including progenitor and stem cells. As we have outlined before, many of these cell populations are likely to be involved in tissue homeostasis, and their behavior might be different under pathological circumstances.[10] Molecular targeting in regenerative medicine is, therefore, a promising area that is rapidly evolving but that faces new challenges to bridge the gap between bench and bedside.
CLICK HERE for subscription information about this journal.
F. P. Luyten, Laboratory for Skeletal Development and Joint Disorders, Division of Rheumatology, Katholieke Universiteit Leuven, Herestraat 49, B-3000 Leuven, Belgium; E-mail: frank.luyten@uz.kuleuven.be.
© 2009 Nature Publishing Group
Cite this: Osteoimmunology: Wnt Antagonists: For Better or Worse? - Medscape - Aug 01, 2009.




Comments