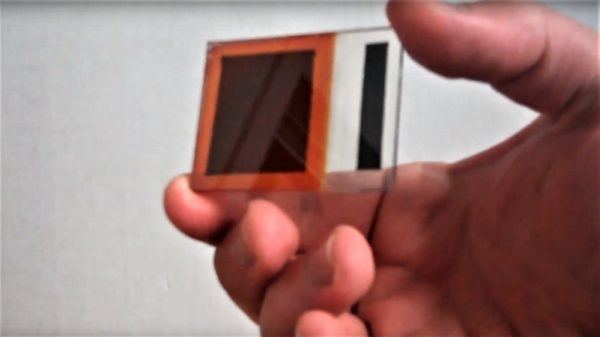
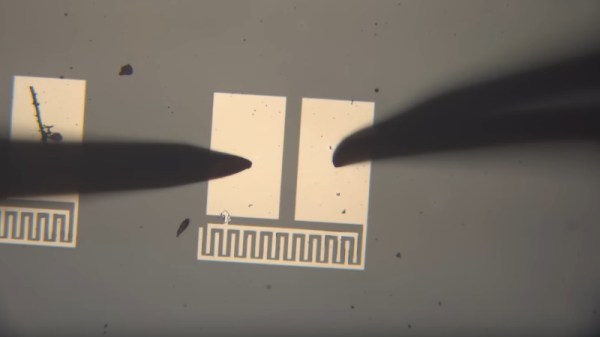
On our travels round the hardware world we’ve encountered more than one group pursuing the goal of making their own silicon integrated circuits, and indeed we’ve seen [Sam Zeloof] producing some of the first practical home-made devices. But silicon is simply one of many different semiconductor materials, and it’s possible to make working semiconductor devices in a less complex lab using some of the others. As an example, [Breaking Taps] has been working with copper (II) oxide, producing photodiodes, and coming within touching distance of a working matrix array.
The video below the break is a comprehensive primer on simple semiconductor production and the challenges of producing copper (II) oxide rather than the lower temperature copper (I) oxide. The devices made have a Schottky junction between the semiconductor and an aluminium conductor, and after some concerns about whether the silicon substrate is part of the circuit and even some spectacular destruction of devices, he has a working photodiode with a satisfying change on the curve tracer when light is applied. The finale is an array of the devices to form a rudimentary camera sensor, but sadly due to alignment issues it’s not quite there yet. We look forward to seeing it when he solves those problems.
As we’ve seen before, copper oxide isn’t the only semiconductor material outside the silicon envelope.
Continue reading “Almost Making A Camera Sensor From Scratch”


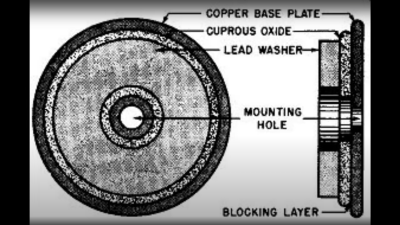 based not upon silicon or germanium, but copper. Copper (I) Oxide is a naturally occurring P-type semiconductor, which can be easily constructed by heating a copper sheet in a flame, and scraping off the outer layer of Copper (II) Oxide leaving the active layer below. Simply making contact to a piece of steel is sufficient to complete the device.
based not upon silicon or germanium, but copper. Copper (I) Oxide is a naturally occurring P-type semiconductor, which can be easily constructed by heating a copper sheet in a flame, and scraping off the outer layer of Copper (II) Oxide leaving the active layer below. Simply making contact to a piece of steel is sufficient to complete the device.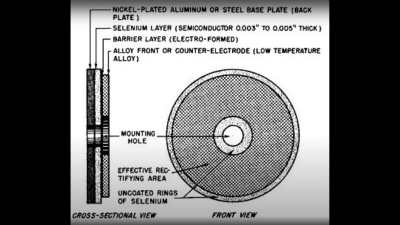 as the Selenium rectifier, based on the properties of a Cadmium Selenide – Selenium interface, which forms an NP junction, albeit one that can’t handle as much power as good old copper. One final device, which was a bit of an improvement upon the original CuO rectifiers, was based upon a stack of Copper Sulphide/Magnesium metal plates, but they came along too late. Once we discovered the wonders of germanium and silicon, it was consigned to the history books before it really saw wide adoption.
as the Selenium rectifier, based on the properties of a Cadmium Selenide – Selenium interface, which forms an NP junction, albeit one that can’t handle as much power as good old copper. One final device, which was a bit of an improvement upon the original CuO rectifiers, was based upon a stack of Copper Sulphide/Magnesium metal plates, but they came along too late. Once we discovered the wonders of germanium and silicon, it was consigned to the history books before it really saw wide adoption.