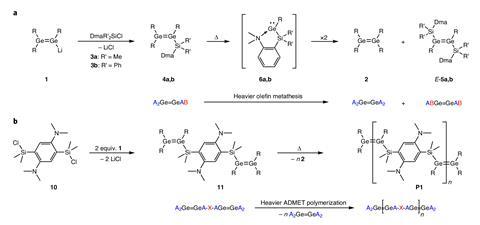
Researchers in Germany have extended the famous olefin metathesis reaction to compounds containing germanium–germanium double bonds.
The metathesis reaction is one of the most widely used reactions in organic synthesis. Its pioneers Yves Chauvin, Robert Grubbs and Richard Schrock shared the 2005 Nobel prize for developing the method.
But while most chemists will be familiar with the reaction in which compounds containing carbon–carbon double bonds exchange partners, fewer will be aware of metathesis reactions involving other species. Most other examples involve small scale reactions of compounds containing phosphorus double bonds, but now a team at Saarland University has demonstrated ‘preparatively useful’ metathesis reactions of digermene compounds.
The group, led by David Scheschkewitz, identified digermene compounds as potential candidates for metathesis reactions due to their tendency to split into respective germylene fragments. Using a specially designed stabilising ligand, the researchers showed that these systems can also undergo the famous shuffling reaction to produce new molecular species, as well as polymers containing repeating germanium–germanium double bond units. Because of the relative weakness of the germanium–germanium double bond, the metathesis reaction can be induced by heating without requiring a catalyst to drive the process.
References
L Klemmer et al, Nat. Chem., 2021, DOI: 10.1038/s41557-021-00639-9














No comments yet