Glaucoma Treatment Strategies: Beyond Lowering IOP
Currently, pharmacological treatments aimed at lowering IOP are the treatments of choice in healthcare systems around the world due to their baroprotective effects.[17] Similarly, surgical intervention is used to either increase the drainage rate of aqueous humor or reduce its production. One common surgical approach is the removal of a portion of the TM (trabeculectomy) or creating pores in the TM using a laser (trabeculoplasty) to increase the drainage rate, reducing IOP. The drawback of surgical procedures is that they are invasive and may not be suitable for patients with co-existing ocular diseases. Additionally, pharmacological and surgical treatments do not address the neurological deterioration that causes vision loss, and often need to be carried out repeatedly due to recurring increases in IOP.[18]
The efficiency of alternative drug-based neuroprotective glaucoma treatments that target apoptosis and inflammatory pathways has been widely investigated (see [11,19,20]). For instance, the prevention of oxidative damage to RGCs due to hypoxia has shown potential for improving neuronal survival. Additionally, melatonin, a free radical scavenger, and Gingko biloba extract were demonstrated as promising drugs for the management of glaucoma through their oxidative-stress relieving effects.[21] Similarly, anti-inflammatory drugs targeting the TNF-α signaling pathway have also been shown to provide neuroprotection. For example, glatiramer acetate, a synthetic analogue of myelin basic protein (a US FDA-approved drug for the treatment of multiple sclerosis), produces a neuroprotective immune response.[22] Alternatively, reducing the production of amyloid β (Aβ) deposits, a major component of plaques associated with glaucoma and Alzheimer's disease, has shown potential in protecting RGCs in experimentally induced glaucoma.[23–25] Drugs that inhibit β-secretase, a key enzyme in the production of Aβ, were also shown to inhibit RGC apoptosis in vitro.[24] Finally, the anti-apoptotic effect of all-trans retinoic acid (ATRA) in the retina has also been demonstrated.[26,27] Sakamoto et al. showed that ATRA has a protective effect against N-methyl-D-aspartate-induced apoptosis in the rat retina by reducing cell death in the ganglion cell layer and the inner nuclear layer, a mechanism that is mediated by ERK/MAPK.[27]
Ocular Gene Therapy
Following the death of RGCs in the retina, their spontaneous regeneration is hindered by several barriers, including the presence of scar tissue at the injury site, formation of spatial gaps in neuronal tissue formed during phagocytosis of dying neurons, and failure of existing adult neurons to initiate axonal extension.[28] Therefore, vision protection or restoration is contingent on treatments that encourage neuronal regeneration against these challenges.[29] Gene therapy offers a promising potential to provide neuroprotective and regenerative function, often in the form of delivery of a protective or an anti-apoptotic gene to injured cells. An ideal gene therapy method should have several attributes. First, levels of gene expression should be high enough to promote phenotypic improvement, without causing overexpression-related toxicity. Second, gene expression should be sustained for long periods of time without the need for repeated administration. Third, the therapeutic load should not cause immunogenic or inflammatory responses in the host.[30]
Gene delivery systems for eye diseases range from simple eye drops and ointments to more advanced bio- and nanotechnology-based systems such as nanoparticles, dendrimers, muco-adhesive systems, iontophoresis, ocular inserts and viruses. Generally, these delivery systems can be classified into two types. The first type, viral gene delivery, uses genetically modified viruses designed to deliver a therapeutic transgene. Despite their excellent transfection efficiency, viral gene delivery systems suffer from limitations related to low titers, inability to transfect post-mitotic cells, induction of a strong immune response and limited insert capacity.[31] The second type, nonviral gene delivery, employs diverse cationic lipids and polymers that compact and bind therapeutic DNA into nano-sized particles.[32] Compared to viral gene delivery vectors, nonviral vectors have a number of advantages, including easy and inexpensive manufacturing, reduced immunogenicity and flexibility in the size of the transgene to be delivered. However, the transfection efficiency of nonviral vectors is poor compared with that of viral vectors and needs significant development before it can be used clinically.[33,34]
Neuroprotective Gene Therapy
There is strong evidence from several research groups that delivery of NF genes, such as those expressing brain-derived neurotrophic factor (BDNF),[35] ciliary neurotrophic factor,[36] glial cell line-derived neurotrophic factor,[37] neurotrophin-4 and sciatic nerve-derived factors,[38] increases the survival of RGCs in rodent models. Among the NFs, BDNF appears to provide the highest level of protection by providing both protective and regenerative functions through direct effects on RGCs by correcting the problems with bidirectional transport of NFs, by indirect influence on other retinal cells and by directing damaged axons. This has been suggested by studies involving adenovirus-mediated intravitreal delivery targeted toward Müller cells,[35] for example, after a single intravitreal injection of BDNF in a cat model.[39] Expression of BDNF also appears to support axonal pathfinding to the brain.[40,41]
Induction of heat shock proteins (HSPs) has also demonstrated effective neuroprotective abilities in RGCs, both in vivo and in vitro.[42,43] Recently, induction of HSPs by local magnetic hyperthermia, was achieved using engineered superparamagnetic nanoparticles in rat retinas.[44] Toxicity assessments of this nanoparticle have also established its feasibility as a physiologically tolerable ocular neuroprotection tool. The apoptosis cascade appears to coincide with activation of cysteine proteases (caspases), therefore delivering genes encoding caspase inhibitors or Bcl-2,[45] an apoptosis inhibitor, and providing protection. Delivery of the BIRC-4 gene in an adeno-associated virus vector by intravitreal injection in a rat chronic glaucoma model, resulted in a decrease in RGC apoptosis through mechanisms that may involve direct inhibition of caspase-3 and -8 or indirect stimulation of neurotrophin production by Müller cells, or both.[46]
Injected versus Needle-free Gene Delivery
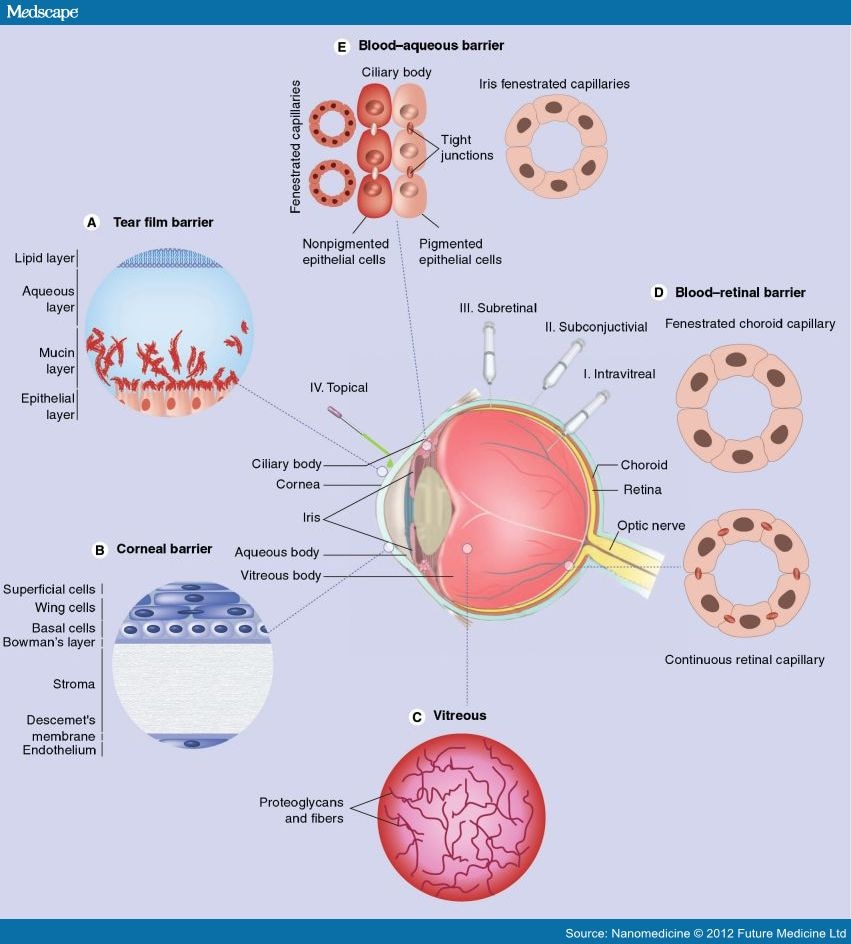
In addition to developing biocompatible and efficacious gene delivery vectors, choosing an appropriate method of delivery is equally important in improving treatment outcomes (Table 1). An ideal route of administration should ensure that the vector remains functional when it reaches the target cell, and that the optimal concentration of the vector is reached in the target tissue with minimal side effects. Generally, the methods used to reach the back of the eye are systemic, intravitreal (Figure 3 I), subconjuctival (Figure 3 II) and subretinal (Figure 3 III) injection. Among these methods, intravitreal and subretinal injections are currently considered to be the most effective and common methods of gene delivery to RGCs. Intravitreal injections are commonly used for delivery to RGCs and inner retinal interneurons. On the other hand, subretinal injections have been shown to be more effective in most cases for delivery to the outer retina. However, these methods are quite invasive and repeated delivery using such methods can cause further complications, such as retinal detachment, hemorrhages, and sub- or pre-retinal fibrosis.[47]
Figure 3.
Biological barriers to topical administration of biotherapeutic molecules into the eye.
Several barriers to topical administration (IV) of drugs to the surface of the eye for delivery to the retina are illustrated. These include the (A) tear film barrier; (B) corneal barrier; (C) vitreous barrier; (D) blood–retinal barrier and (E) blood–aqueous barrier. Several common delivery methods including (I) intravitreal injection; (II) subconjuctival injection and (III) subretinal injection can bypass some of these barriers, but are invasive and can result in further damage to the eye, meanwhile (IV) topical administration in the form of eye drops is a non-invasive delivery method that can be performed repeatedly with minimal side effects.
Adapted from. [116,117]
Parenteral administration allows for the delivery of larger volumes of a delivery vector and also allows for repeated administration. However, the therapeutic effect achieved by these methods is very limited due to factors restricting access to the eye. Even with these challenges, Zhang et al. intravenously delivered 85-nm pegylated immunoliposomes into ocular cells of rhesus monkeys.[48] The delivery was carried out with a monoclonal antibody targeted to the human insulin receptor. This delivery method takes advantage of the insulin receptor that is highly expressed in the outer nuclear layer of the retina and in the blood–retinal barrier. However, such delivery methods can be associated with nonspecific absorption of the transgene by other tissues, which can result in serious toxicity issues.
Topical gene delivery (Figure 3 IV) to the ocular surface would be the safest method since it is noninvasive and painless compared with other delivery methods. However, there are only a few examples of successful attempts to achieve gene delivery to the retina via this route. Liaw et al. reported gene expression around the iris, sclera, conjunctiva and lateral rectus muscle of rabbit eyes and in intraocular tissue of nude mice, following eyedrop administration of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles.[49,50] Two other studies reported successful gene delivery to RGCs after topical administration of liposomes in rats.[51,52] More recently, peptide-for-ocular-delivery nanoparticles were shown to successfully transfect retinal cells after topical administration.[53]
The noninvasive route of administration is perhaps the most ambitious goal since the barriers associated with topical gene delivery to the posterior ocular tissue are the most challenging. First, the tear film (Figure 3A), which is an aqueous layer covered by lipids and underlined by mucin, covers the corneal and conjuctival layers and limits the bioavailability of applied vectors due to tear turnover rate as well as lacrimal and nasolacrimal drainage. Addition of viscosity enhancers, such as cellulose derivatives or thermoreversible poloxamer gels, was attempted to increase bioavailability, however, with little success.[54,55] More successful has been the incorporation of muco-adhesive polymers, such as chitosan and hyaluronic acid derivatives, in gene delivery vehicles.[56] Tissue barriers, namely the cornea (Figure 3B), conjuctiva, sclera and choroid, pose resistance to the passage of vectors due to the presence of epithelial tight junctions, proteoglycan matrices and fibril collagen networks within their structures. The cornea is made up of several layers: a keratinized superficial layer, wing cell layer, basal cell layer, Bowman's membrane, stroma, Descemet's membrane and an endothelium layer.[57] The corneal epithelium is the most limiting to drug delivery due to the presence of tight junctions within its structure, allowing the selective passage of small molecules via the transcellular route.[58] The final barrier limiting both topical and injected delivery of genes to the retina, the vitreous (Figure 3C), a primarily aqueous biogel consisting of collagen, hyaluronan and proteoglycans, was shown to decrease nonviral gene transfer to RGCs. Electrostatic binding of anionic glycoaminoglycans to cationic gene complexes may detach nucleic acid inserts from the complexes or change their charge and size, therefore affecting their transfection efficiency.[59]
Nanomedicine. 2012;7(7):1067-1083. © 2012 Future Medicine Ltd.








Comments