Abstract
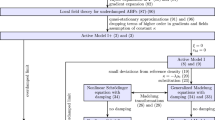
Active matter, which ranges from molecular motors to groups of animals, exists at different length scales and timescales, and various computational models have been proposed to describe and predict its behaviour. The diversity of the methods and the challenges in modelling active matter primarily originate from the out-of-equilibrium character, lack of detailed balance and of time-reversal symmetry, multiscale nature, nonlinearity and multibody interactions. Models exist for both dry active matter and active matter in fluids, and can be agent-based or continuum-level descriptions. They can be generic, emphasizing universal features, or detailed, capturing specific features. We compare various modelling approaches and numerical techniques to illuminate the innovations and challenges in understanding active matter.
Key points
Active matter exhibits a wide range of emergent non-equilibrium phenomena, theoretical studies of which often require computer simulations.
Active matter encompasses synthetic and living systems, including active gels and the cytoskeleton, cells and tissues, nanorobots and microrobots, synthetic and biological microswimmers, and animal herds.
Active matter is characterized by out-of-equilibrium behaviour, nonlinearity, multibody interactions, lack of detailed balance or time-reversal symmetry and, generically, absence of an equation of state.
The wide spectrum of systems and phenomena requires a multitude of models and simulation techniques, from agent-based to continuum-level approaches, and combinations thereof.
Active agents can interact in many ways, such as volume exclusion, contact attraction, visual information and hydrodynamics. Hydrodynamic interactions are ubiquitous for self-propelled particles in an aqueous environment, which implies a classification into dry and wet active matter.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Marchetti, M. C. et al. Hydrodynamics of soft active matter. Rev. Mod. Phys. 85, 1143–1189 (2013). Comprehensive overview of the hydrodynamic theories of active matter.
Ramaswamy, S. The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 1, 323–345 (2010).
Toner, J., Tu, Y. & Ramaswamy, S. Hydrodynamics and phases of flocks. Ann. Phys. 318, 170–244 (2005).
Elgeti, J., Winkler, R. G. & Gompper, G. Physics of microswimmers—single particle motion and collective behavior: a review. Rep. Prog. Phys. 78, 056601 (2015). Review of theories, simulations and experiments on microswimmers.
Bechinger, C. et al. Active particles in complex and crowded environments. Rev. Mod. Phys. 88, 045006 (2016). Guided tour of artificial self-propelling microparticles and nanoparticles, and their application to the study of nonequilibrium phenomena.
Solon, A. P. et al. Pressure is not a state function for generic active fluids. Nat. Phys. 11, 673–678 (2015).
Bialké, J., Speck, T. & Löwen, H. Crystallization in a dense suspension of self-propelled particles. Phys. Rev. Lett. 108, 168301 (2012).
Redner, G. S., Hagan, M. F. & Baskaran, A. Structure and dynamics of a phase-separating active colloidal fluid. Phys. Rev. Lett. 110, 055701 (2013).
Cates, M. E. & Tailleur, J. Motility-induced phase separation. Annu. Rev. Condens. Matter Phys. 6, 219–244 (2015). Review of theoretical descriptions of motility-induced phase separation in active matter.
Wysocki, A., Winkler, R. G. & Gompper, G. Cooperative motion of active Brownian spheres in three-dimensional dense suspensions. Europhys. Lett. 105, 48004 (2014).
Stenhammar, J., Marenduzzo, D., Allen, R. J. & Cates, M. E. Phase behaviour of active Brownian particles: the role of dimensionality. Soft Matter 10, 1489–1499 (2014).
Wysocki, A., Winkler, R. G. & Gompper, G. Propagating interfaces in mixtures of active and passive Brownian particles. New J. Phys. 18, 123030 (2016).
Stenhammar, J., Wittkowski, R., Marenduzzo, D. & Cates, M. E. Activity-induced phase separation and self-assembly in mixtures of active and passive particles. Phys. Rev. Lett. 114, 018301 (2015).
Digregorio, P. et al. Full phase diagram of active Brownian disks: from melting to motility-induced phase separation. Phys. Rev. Lett. 121, 098003 (2018).
Fily, Y., Henkes, S. & Marchetti, M. C. Freezing and phase separation of self-propelled disks. Soft Matter 10, 2132–2140 (2014).
Elgeti, J. & Gompper, G. Wall accumulation of self-propelled spheres. EPL 101, 48003 (2013).
Fily, Y., Baskaran, A. & Hagan, M. Dynamics of self-propelled particles under strong confinement. Soft Matter 10, 5609–5617 (2014).
Das, S., Gompper, G. & Winkler, R. G. Local stress and pressure in an inhomogeneous system of spherical active Brownian particles. Sci. Rep. 9, 6608 (2019).
Wysocki, A. & Rieger, H. Capillary action in scalar active matter. Phys. Rev. Lett. 124, 048001 (2019).
Takatori, S. C., Yan, W. & Brady, J. F. Swim pressure: stress generation in active matter. Phys. Rev. Lett. 113, 028103 (2014).
Winkler, R. G., Wysocki, A. & Gompper, G. Virial pressure in systems of active Brownian particles. Soft Matter 11, 6680–6691 (2015).
Fily, Y., Kafri, Y., Solon, A. P., Tailleur, J. & Turner, A. Mechanical pressure and momentum conservation in dry active matter. J. Phys. A 51, 044003 (2018).
Wysocki, A., Elgeti, J. & Gompper, G. Giant adsorption of microswimmers: duality of shape asymmetry and wall curvature. Phys. Rev. E 91, 050302(R) (2015).
Romanczuk, P., Bär, M., Ebeling, W., Lindner, B. & Schimansky-Geier, L. Active Brownian particles. Eur. Phys. J. Spec. Top. 202, 1–162 (2012).
Nguyen, N. H. P., Klotsa, D., Engel, M. & Glotzer, S. C. Emergent collective phenomena in a mixture of hard shapes through active rotation. Phys. Rev. Lett. 112, 075701 (2014).
Löwen, H. Chirality in microswimmer motion: from circle swimmers to active turbulence. Eur. Phys. J. Spec. Top. 225, 2319–2331 (2016).
Peruani, F. Active Brownian rods. Eur. Phys. J. Spec. Top. 225, 2301–2317 (2016).
ten Hagen, B. et al. Can the self-propulsion of anisotropic microswimmers be described by using forces and torques? J. Phys. Condens. Matter 27, 194110 (2015).
Kaiser, A., Babel, S., ten Hagen, B., von Ferber, C. & Löwen, H. How does a flexible chain of active particles swell? J. Chem. Phys. 142, 124905 (2015).
Eisenstecken, T., Gompper, G. & Winkler, R. G. Conformational properties of active semiflexible polymers. Polymers 8, 304 (2016).
Eisenstecken, T., Gompper, G. & Winkler, R. G. Internal dynamics of semiflexible polymers with active noise. J. Chem. Phys. 146, 154903 (2017).
Kourbane-Houssene, M., Erignoux, C., Bodineau, T. & Tailleur, J. Exact hydrodynamic description of active lattice gases. Phys. Rev. Lett. 120, 268003 (2018).
Klamser, J. U., Kapfer, S. C. & Krauth, W. Thermodynamic phases in two-dimensional active matter. Nat. Commun. 9, 5045 (2018).
Sadjadi, Z., Shaebani, M. R., Rieger, H. & Santen, L. Persistent-random-walk approach to anomalous transport of self-propelled particles. Phys. Rev. E 91, 062715 (2015).
Shaebani, M. R., Sadjadi, Z., Sokolov, I. M., Rieger, H. & Santen, L. Anomalous diffusion of self-propelled particles in directed random environments. Phys. Rev. E 90, 030701 (2014).
Levis, D. & Berthier, L. Clustering and heterogeneous dynamics in a kinetic Monte Carlo model of self-propelled hard disks. Phys. Rev. E 89, 062301 (2014).
Najafi, J. et al. Flagellar number governs bacterial spreading and transport efficiency. Sci. Adv. 4, eaar6425 (2018).
Hafner, A. E., Santen, L., Rieger, H. & Shaebani, M. R. Run-and-pause dynamics of cytoskeletal motor proteins. Sci. Rep. 6, 37162 (2016).
Vicsek, T. & Zafeiris, A. Collective motion. Phys. Rep. 517, 71–140 (2012). Review of collective motion of active agents, from macromolecules through metallic rods and robots to groups of animals and people.
Vicsek, T., Czirók, A., Ben-Jacob, E., Cohen, I. & Shochet, O. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229 (1995).
Solon, A. P., Chaté, H. & Tailleur, J. From phase to microphase separation in flocking models: the essential role of nonequilibrium fluctuations. Phys. Rev. Lett. 114, 068101 (2015).
Solon, A. P. & Tailleur, J. Revisiting the flocking transition using active spins. Phys. Rev. Lett. 111, 078101 (2013).
Ginelli, F., Peruani, F., Bär, M. & Chaté, H. Large-scale collective properties of self-propelled rods. Phys. Rev. Lett. 104, 184502 (2010).
Chaté, H., Ginelli, F., Grégoire, G., Peruani, F. & Raynaud, F. Modeling collective motion: variations on the vicsek model. Eur. Phys. J. B 64, 451–456 (2008).
Strömbom, D. Collective motion from local attraction. J. Theor. Biol. 283, 145–151 (2011).
Aldana, M., Dossetti, V., Huepe, C., Kenkre, V. M. & Larralde, H. Phase transitions in systems of self-propelled agents and related network models. Phys. Rev. Lett. 98, 095702 (2007).
Aldana, M., Larralde, H. & Vazquez, B. On the emergence of collective order in swarming systems: a recent debate. Int. J. Mod. Phys. B 23, 3661–3685 (2009).
Peruani, F. & Aranson, I. S. Cold active motion: how time-independent disorder affects the motion of self-propelled agents. Phys. Rev. Lett. 120, 238101 (2018).
Grossman, D., Aranson, I. S. & Jacob, E. B. Emergence of agent swarm migration and vortex formation through inelastic collisions. New J. Phys. 10, 023036 (2008).
Nagy, M., Daruka, I. & Vicsek, T. New aspects of the continuous phase transition in the scalar noise model (snm) of collective motion. Physica A 373, 445–454 (2007).
Peruani, F., Klauss, T., Deutsch, A. & Voss-Boehme, A. Traffic jams, gliders, and bands in the quest for collective motion of self-propelled particles. Phys. Rev. Lett. 106, 128101 (2011).
Ginelli, F. & Chaté, H. Relevance of metric-free interactions in flocking phenomena. Phys. Rev. Lett. 105, 168103 (2010).
Gregoire, G., Chaté, H. & Tu, Y. Moving and staying together without a leader. Physica D 181, 157–170 (2003).
Szabó, P., Nagy, M. & Vicsek, T. Transitions in a self-propelled-particles model with coupling of accelerations. Phys. Rev. E 79, 021908 (2009).
Chaté, H., Ginelli, F. & Montagne, R. Simple model for active nematics: quasi-long-range order and giant fluctuations. Phys. Rev. Lett. 96, 180602 (2006).
Mahault, B. et al. Self-propelled particles with velocity reversals and ferromagnetic alignment: active matter class with second-order transition to quasi-long-range polar order. Phys. Rev. Lett. 120, 258002 (2018).
Szabó, B. et al. Phase transition in the collective migration of tissue cells: experiment and model. Phys. Rev. E 74, 061908 (2006).
Peruani, F., Deutsch, A. & Bär, M. Nonequilibrium clustering of self-propelled rods. Phys. Rev. E 74, 030904 (2006).
Toner, J. & Tu, Y. Long-range order in a two-dimensional dynamical XY model: how birds fly together. Phys. Rev. Lett. 75, 4326–4329 (1995).
Toner, J. & Tu, Y. Flocks, herds, and schools: a quantitative theory of flocking. Phys. Rev. E 58, 4828–4858 (1998).
Bertin, E., Droz, M. & Grégoire, G. Boltzmann and hydrodynamic description for self-propelled particles. Phys. Rev. E 74, 022101 (2006).
Ihle, T. Kinetic theory of flocking: derivation of hydrodynamic equations. Phys. Rev. E 83, 030901 (2011).
Ranft, J. et al. Fluidization of tissues by cell division and apoptosis. Proc. Natl Acad. Sci. USA 107, 20863–20868 (2010).
Narayan, V., Ramaswamy, S. & Menon, N. Long-lived giant number fluctuations in a swarming granular nematic. Science 317, 105–108 (2007).
Ramaswamy, S., Simha, R. A. & Toner, J. Active nematics on a substrate: giant number fluctuations and long-time tails. EPL 62, 196–202 (2003).
Hemingway, E. J. et al. Active viscoelastic matter: from bacterial drag reduction to turbulent solids. Phys. Rev. Lett. 114, 098302 (2015).
Schwarz, U. S. & Safran, S. A. Physics of adherent cells. Rev. Mod. Phys. 85, 1327–1381 (2013).
Hohenberg, P. C. & Halperin, B. I. Theory of dynamic critical phenomena. Rev. Mod. Phys. 49, 435–479 (1977).
Wittkowski, R. et al. Scalar \({\phi }^{4}\) field theory for active-particle phase separation. Nat. Commun. 5, 4351 (2014).
Tjhung, E., Nardini, C. & Cates, M. E. Cluster phases and bubbly phase separation in active fluids: reversal of the ostwald process. Phys. Rev. X 8, 031080 (2018).
Stenhammar, J., Tiribocchi, A., Allen, R. J., Marenduzzo, D. & Cates, M. E. Continuum theory of phase separation kinetics for active Brownian particles. Phys. Rev. Lett. 111, 145702 (2013).
Cates, M. E. & Tailleur, J. When are active Brownian particles and run-and-tumble particles equivalent? Consequences for motility-induced phase separation. EPL 101, 20010 (2013).
Tailleur, J. & Cates, M. E. Statistical mechanics of interacting run-and-tumble bacteria. Phys. Rev. Lett. 100, 218103 (2008).
Purcell, E. M. Life at low Reynolds number. Am. J. Phys. 45, 3–11 (1977).
Spagnolie, S. E. & Lauga, E. Hydrodynamics of self-propulsion near a boundary: predictions and accuracy of far-field approximations. J. Fluid Mech. 700, 105–147 (2012).
Winkler, R. G. & Gompper, G. in Handbook of Materials Modeling: Methods: Theory and Modeling (eds Andreoni, W. & Yip, S.) 1–20 (Springer, 2018).
Berke, A. P., Turner, L., Berg, H. C. & Lauga, E. Hydrodynamic attraction of swimming microorganisms by surfaces. Phys. Rev. Lett. 101, 038102 (2008).
Lauga, E. & Powers, T. R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 096601 (2009).
Elgeti, J. & Gompper, G. Microswimmers near surfaces. Eur. Phys. J. Spec. Top. 225, 2333–2352 (2016).
Li, G. & Tang, J. X. Accumulation of microswimmers near a surface mediated by collision and rotational Brownian motion. Phys. Rev. Lett. 103, 078101 (2009).
Elgeti, J. & Gompper, G. Self-propelled rods near surfaces. EPL 85, 38002 (2009).
Elgeti, J. & Gompper, G. Run-and-tumble dynamics of self-propelled particles in confinement. EPL 109, 58003 (2015).
Schaar, K., Zöttl, A. & Stark, H. Detention times of microswimmers close to surfaces: influence of hydrodynamic interactions and noise. Phys. Rev. Lett. 115, 038101 (2015).
Saintillan, D. & Shelley, M. J. Orientational order and instabilities in suspensions of self-locomoting rods. Phys. Rev. Lett. 99, 058102 (2007).
Saintillan, D. & Shelley, M. J. Instabilities and pattern formation in active particle suspensions: kinetic theory and continuum simulations. Phys. Rev. Lett. 100, 178103 (2008).
Sanchez, T., Chen, D. T. N., DeCamp, S. J., Heymann, M. & Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 491, 431–434 (2012).
Thampi, S. P., Golestanian, R. & Yeomans, J. M. Velocity correlations in an active nematic. Phys. Rev. Lett. 111, 118101 (2013).
Giomi, L., Bowick, M. J., Ma, X. & Marchetti, M. C. Defect annihilation and proliferation in active nematics. Phys. Rev. Lett. 110, 228101 (2013).
Keber, F. C. et al. Topology and dynamics of active nematic vesicles. Science 345, 1135–1139 (2014).
Mathijssen, A. J. T. M., Culver, J., Bhamla, M. S. & Prakash, M. Collective intercellular communication through ultra-fast hydrodynamic trigger waves. Nature 571, 560–564 (2019).
Qiu, T. et al. Swimming by reciprocal motion at low Reynolds number. Nat. Commun. 5, 5119 (2014).
Qin, B., Gopinath, A., Yang, J., Gollub, J. P. & Arratia, P. E. Flagellar kinematics and swimming of algal cells in viscoelastic fluids. Sci. Rep. 5, 9190 (2015).
Patteson, A. E., Gopinath, A., Goulian, M. & Arratia, P. E. Running and tumbling with E. coli in polymeric solutions. Sci. Rep. 5, 15761 (2015).
Li, G. & Ardekani, A. M. Collective motion of microorganisms in a viscoelastic fluid. Phys. Rev. Lett. 117, 118001 (2016).
Lauga, E. Propulsion in a viscoelastic fluid. Phys. Fluids 19, 083104 (2007).
Fu, H. C., Wolgemuth, C. W. & Powers, T. R. Swimming speeds of filaments in nonlinearly viscoelastic fluids. Phys. Fluids 21, 033102 (2009).
Spagnolie, S. E., Liu, B. & Powers, T. R. Locomotion of helical bodies in viscoelastic fluids: enhanced swimming at large helical amplitudes. Phys. Rev. Lett. 111, 068101 (2013).
Man, Y. & Lauga, E. Phase-separation models for swimming enhancement in complex fluids. Phys. Rev. E 92, 023004 (2015).
Liu, B., Powers, T. R. & Breuer, K. S. Force-free swimming of a model helical flagellum in viscoelastic fluids. Proc. Natl Acad. Sci. USA 108, 19516–19520 (2011).
Gagnon, D. A., Keim, N. C. & Arratia, P. E. Undulatory swimming in shear-thinning fluids: experiments with Caenorhabditis elegans. J. Fluid Mech. 758, R3 (2014).
Martinez, V. A. et al. Flagellated bacterial motility in polymer solutions. Proc. Natl Acad. Sci. USA 111, 17771–17776 (2014).
Zöttl, A. & Yeomans, J. M. Enhanced bacterial swimming speeds in macromolecular polymer solutions. Nat. Phys. 15, 554–558 (2019).
McNamara, G. R. & Zanetti, G. Use of the Boltzmann equation to simulate lattice-gas automata. Phys. Rev. Lett. 61, 2332–2335 (1988).
Dünweg, B. & Ladd, A. J. C. Lattice Boltzmann simulations of soft matter systems. Adv. Polym. Sci. 221, 89–166 (2009).
Español, P. & Warren, P. Statistical mechanics of dissipative particle dynamics. EPL 30, 191–196 (1995).
Kapral, R. Advances in Chemical Physics (Wiley, 2008).
Gompper, G., Ihle, T., Kroll, D. M. & Winkler, R. G. Multi-particle collision dynamics: a particle-based mesoscale simulation approach to the hydrodynamics of complex fluids. Adv. Polym. Sci. 221, 1–87 (2009).
Ishikawa, T. & Pedley, T. J. Coherent structures in monolayers of swimming particles. Phys. Rev. Lett. 100, 088103 (2008).
Mathijssen, A. J. T. M., Doostmohammadi, A., Yeomans, J. M. & Shendruk, T. N. Hydrodynamics of micro-swimmers in films. J. Fluid Mech. 806, 35–70 (2016).
Singh, R., Ghose, S. & Adhikari, R. Many-body microhydrodynamics of colloidal particles with active boundary layers. J. Stat. Mech. Theor. Exp. 2015, P06017 (2015).
Elgeti, J., Kaupp, U. B. & Gompper, G. Hydrodynamics of sperm cells near surfaces. Biophys. J. 99, 1018–1026 (2010).
Hu, J., Yang, M., Gompper, G. & Winkler, R. G. Modelling the mechanics and hydrodynamics of swimming E. coli. Soft Matter 11, 7867–7876 (2015).
Watari, N. & Larson, R. G. The hydrodynamics of a run-and-tumble bacterium propelled by polymorphic helical flagella. Biophys. J. 98, 12–17 (2010).
Shum, H.,Gaffney, E. A. & Smith D. J. Modelling bacterial behaviour close to a no-slip plane boundary: the influence of bacterial geometry. Proc. R. Soc. A 466, 1725–1748 (2010).
Pimponi, D., Chinappi, M., Gualtieri, P. & Casciola, C. M. Hydrodynamics of flagellated microswimmers near free-slip interfaces. J. Fluid Mech. 789, 514–533 (2016).
Lighthill, M. J. On the squirming motion of nearly spherical deformable bodies through liquids at very small Reynolds numbers. Comm. Pure Appl. Math. 5, 109–118 (1952).
Blake, J. R. A spherical envelope approach to ciliary propulsion. J. Fluid Mech. 46, 199–208 (1971).
Ishikawa, T., Simmonds, M. P. & Pedley, T. J. Hydrodynamic interaction of two swimming model micro-organisms. J. Fluid Mech. 568, 119–160 (2006).
Pedley, T. J. Spherical squirmers: models for swimming micro-organisms. IMA J. Appl. Math. 81, 488–521 (2016).
Llopis, I. & Pagonabarraga, I. Hydrodynamic interactions in squirmer motion: swimming with a neighbour and close to a wall. J. Nonnewton. Fluid Mech. 165, 946–952 (2010).
Götze, I. O. & Gompper, G. Mesoscale simulations of hydrodynamic squirmer interactions. Phys. Rev. E 82, 041921 (2010).
Evans, A. A., Ishikawa, T., Yamaguchi, T. & Lauga, E. Orientational order in concentrated suspensions of spherical microswimmers. Phys. Fluids 23, 111702 (2011).
Alarcon, F. & Pagonabarraga, I. Spontaneous aggregation and global polar ordering in squirmer suspensions. J. Mol. Liq. 185, 56–61 (2013).
Molina, J. J., Nakayama, Y. & Yamamoto, R. Hydrodynamic interactions of self-propelled swimmers. Soft Matter 9, 4923–4936 (2013).
Yoshinaga, N. & Liverpool, T. B. Hydrodynamic interactions in dense active suspensions: from polar order to dynamical clusters. Phys. Rev. E 96, 020603 (2017).
Ishimoto, K. & Gaffney, E. A. Squirmer dynamics near a boundary. Phys. Rev. E 88, 062702 (2013).
Lintuvuori, J. S., Brown, A. T., Stratford, K. & Marenduzzo, D. Hydrodynamic oscillations and variable swimming speed in squirmers close to repulsive walls. Soft Matter 12, 7959–7968 (2016).
Theers, M., Westphal, E., Gompper, G. & Winkler, R. G. Modeling a spheroidal microswimmer and cooperative swimming in a narrow slit. Soft Matter 12, 7372–7385 (2016).
Theers, M., Westphal, E., Qi, K., Winkler, R. G. & Gompper, G. Clustering of microswimmers: interplay of shape and hydrodynamics. Soft Matter 14, 8590–8603 (2018).
Keller, S. R. & Wu, T. Y. A porous prolate-spheroidal model for ciliated micro-organisms. J. Fluid Mech. 80, 259–278 (1977).
Theers, M., Westphal, E., Gompper, G. & Winkler, R. G. From local to hydrodynamic friction in Brownian motion: a multiparticle collision dynamics simulation study. Phys. Rev. E 93, 032604 (2016).
Nash, R. W., Adhikari, R., Tailleur, J. & Cates, M. E. Run-and-tumble particles with hydrodynamics: sedimentation, trapping, and upstream swimming. Phys. Rev. Lett. 104, 258101 (2010).
Hernandez-Ortiz, J. P., Stoltz, C. G. & Graham, M. D. Transport and collective dynamics in suspensions of confined swimming particles. Phys. Rev. Lett. 95, 204501 (2005).
de Graaf, J. et al. Lattice Boltzmann hydrodynamics of anisotropic active matter. J. Chem. Phys. 144, 134106 (2016).
Menzel, A. M., Saha, A., Hoell, C. & Löwen, H. Dynamical density functional theory for microswimmers. J. Chem. Phys. 144, 024115 (2016).
Lighthill, J. Flagellar hydrodynamics. SIAM Rev. 18, 161–230 (1976).
Saggiorato, G. et al. Human sperm steer with second harmonics of the flagellar beat. Nat. Commun. 8, 1415 (2017).
Shum, H. & Gaffney, E. A. Hydrodynamic analysis of flagellated bacteria swimming near one and between two no-slip plane boundaries. Phys. Rev. E 91, 033012 (2015).
Lauga, E. Bacterial hydrodynamics. Annu. Rev. Fluid Mech. 48, 105–130 (2016). Comprehensive review of the hydrodynamics of bacteria.
Rode, S., Elgeti, J. & Gompper, G. Sperm motility in modulated microchannels. New J. Phys. 21, 013016 (2019).
Reigh, S. Y., Winkler, R. G. & Gompper, G. Synchronization and bundling of anchored bacterial flagella. Soft Matter 8, 4363–4372 (2012).
Reichert, M. & Stark, H. Synchronization of rotating helices by hydrodynamic interactions. Eur. Phys. J. E 17, 493–500 (2005).
Vogel, R. & Stark, H. Motor-driven bacterial flagella and buckling instabilities. Eur. Phys. J. E 35, 15 (2012).
Janssen, P. J. A. & Graham, M. D. Coexistence of tight and loose bundled states in a model of bacterial flagellar dynamics. Phys. Rev. E 84, 011910 (2011).
Hu, J., Wysocki, A., Winkler, R. G. & Gompper, G. Physical sensing of surface properties by microswimmers - directing bacterial motion via wall slip. Sci. Rep. 5, 9586 (2015).
Lemelle, L., Palierne, J.-F., Chatre, E., Vaillant, C. & Place, C. Curvature reversal of the circular motion of swimming bacteria probes for slip at solid/liquid interfaces. Soft Matter 9, 9759–9762 (2013).
Lauga, E., DiLuzio, W. R., Whitesides, G. M. & Stone, H. A. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90, 400–412 (2006).
Di Leonardo, R., Dell Arciprete, D., Angelani, L. & Iebba, V. Swimming with an image. Phys. Rev. Lett. 106, 038101 (2011).
Dombrowski, C., Cisneros, L., Chatkaew, S., Goldstein, R. E. & Kessler, J. O. Self-concentration and large-scale coherence in bacterial dynamics. Phys. Rev. Lett. 93, 098103 (2004).
Matas-Navarro, R., Golestanian, R., Liverpool, T. B. & Fielding, S. M. Hydrodynamic suppression of phase separation in active suspensions. Phys. Rev. E 90, 032304 (2014).
Gaspard, P. & Kapral, R. Thermodynamics and statistical mechanics of chemically powered synthetic nanomotors. Adv. Phys. X 4, 1602480 (2019).
Bayati, P., Popescu, M. N., Uspal, W. E., Dietrich, S. & Najafi, A. Dynamics near planar walls for various model self-phoretic particles. Soft Matter 15, 5644–5672 (2019).
Rückner, G. & Kapral, R. Chemically powered nanodimers. Phys. Rev. Lett. 98, 150603 (2007).
Yang, M. & Ripoll, M. Simulations of thermophoretic nanoswimmers. Phys. Rev. E 84, 061401 (2011).
Saha, S., Golestanian, R. & Ramaswamy, S. Clusters, asters, and collective oscillations in chemotactic colloids. Phys. Rev. E 89, 062316 (2014).
Michelin, S. & Lauga, E. Phoretic self-propulsion at finite Péclet numbers. J. Fluid Mech. 747, 572 (2014).
Liebchen, B., Marenduzzo, D., Pagonabarraga, I. & Cates, M. E. Clustering and pattern formation in chemorepulsive active colloids. Phys. Rev. Lett. 115, 258301 (2015).
Stark, H. Artificial chemotaxis of self-phoretic active colloids: collective behavior. Acc. Chem. Res. 51, 2681–2688 (2018).
Moran, J. L. & Posner, J. D. Phoretic self-propulsion. Annu. Rev. Fluid Mech. 49, 511–540 (2017).
Howse, J. R. et al. Self-motile colloidal particles: from directed propulsion to random walk. Phys. Rev. Lett. 99, 048102 (2007).
Uspal, W. E., Popescu, M. N., Dietrich, S. & Tasinkevych, M. Self-propulsion of a catalytically active particle near a planar wall: from reflection to sliding and hovering. Soft Matter 11, 434 (2015).
Ishimoto, K. & Gaffney, E. A. Fluid flow and sperm guidance: a simulation study of hydrodynamic sperm rheotaxis. J. Roy. Soc. Interface 12, 20150172 (2015).
Koh, J. B. Y., Shen, X. & Marcos. Theoretical modeling in microscale locomotion. Microfluid. Nanofluid. 20, 98 (2016).
Uspal, W. E., Popescu, M. N., Dietrich, S. & Tasinkevych, M. Rheotaxis of spherical active particles near a planar wall. Soft Matter 11, 6613–6632 (2015).
Mathijssen, A. et al. Oscillatory surface rheotaxis of swimming E. coli bacteria. Nat. Commun. 20, 3434 (2019).
Friedrich, B. M. & Jülicher, F. Chemotaxis of sperm cells. Proc. Natl Acad. Sci. USA 104, 13256 (2007).
Tu, Y. Quantitative modeling of bacterial chemotaxis: signal amplification and accurate adaptation. Annu. Rev. Biophys. 42, 337–359 (2013).
Camley, B. A., Zimmermann, J., Levine, H. & Rappel, W.-J. Emergent collective chemotaxis without single-cell gradient sensing. Phys. Rev. Lett. 116, 098101 (2016).
ten Hagen, B. et al. Gravitaxis of asymmetric self-propelled colloidal particles. Nat. Commun. 5, 4829 (2014).
Kuhr, J.-T., Blaschke, J., Rühle, F. & Stark, H. Collective sedimentation of squirmers under gravity. Soft Matter 13, 7548–7555 (2017).
Cohen, J. A. & Golestanian, R. Emergent cometlike swarming of optically driven thermally active colloids. Phys. Rev. Lett. 112, 068302 (2014).
Martin, P. C., Parodi, O. & Pershan, P. S. Unified hydrodynamic theory for crystals, liquid crystals, and normal fluids. Phys. Rev. A 6, 2401–2420 (1972).
Prost, J., Jülicher, F. & Joanny, J.-F. Active gel phys. Nat. Phys. 11, 111–117 (2015). Introduction to active-gel models for actomyosin.
Jülicher, F., Grill, S. W. & Salbreux, G. Hydrodynamic theory of active matter. Rep. Prog. Phys. 81, 076601 (2018).
Carenza, L. N., Gonnella, G., Lamura, A., Negro, G. & Tiribocchi, A. Lattice Boltzmann methods and active fluids. Eur. Phys. J. E 42, 81 (2019).
AditiSimha, R. & Ramaswamy, S. Hydrodynamic fluctuations and instabilities in ordered suspensions of self-propelled particles. Phys. Rev. Lett. 89, 058101 (2002).
Hatwalne, Y., Ramaswamy, S., Rao, M. & Simha, R. A. Rheology of active-particle suspensions. Phys. Rev. Lett. 92, 118101 (2004).
Kruse, K., Joanny, J. F., Jülicher, F., Prost, J. & Sekimoto, K. Asters, vortices, and rotating spirals in active gels of polar filaments. Phys. Rev. Lett. 92, 078101 (2004).
Giomi, L., Marchetti, M. C. & Liverpool, T. B. Complex spontaneous flows and concentration banding in active polar films. Phys. Rev. Lett. 101, 198101 (2008).
Baskaran, A. & Marchetti, M. C. Statistical mechanics and hydrodynamics of bacterial suspensions. Proc. Natl Acad. Sci. USA 106, 15567–15572 (2009).
Linkmann, M., Marchetti, M. C., Boffetta, G. & Eckhardt, B. Condensate formation and multiscale dynamics in two-dimensional active suspensions. Preprint at arXiv https://arxiv.org/abs/1905.06267 (2019).
Marenduzzo, D., Orlandini, E., Cates, M. E. & Yeomans, J. M. Steady-state hydrodynamic instabilities of active liquid crystals: hybrid lattice Boltzmann simulations. Phys. Rev. E 76, 031921 (2007).
Marenduzzo, D., Orlandini, E. & Yeomans, J. M. Hydrodynamics and rheology of active liquid crystals: a numerical investigation. Phys. Rev. Lett. 98, 118102 (2007).
Giomi, L., Mahadevan, L., Chakraborty, B. & Hagan, M. F. Excitable patterns in active nematics. Phys. Rev. Lett. 106, 218101 (2011).
Doostmohammadi, A., Ignes-Mullol, J., Yeomans, J. M. & Sagues, F. Active nematics. Nat. Commun. 9, 3246 (2018).
Dunkel, J., Heidenreich, S., Bär, M. & Goldstein, R. E. Minimal continuum theories of structure formation in dense active fluids. New J. Phys. 15, 045016 (2013).
Slomka, J. & Dunkel, J. Generalized Navier-Stokes equations for active suspensions. Eur. Phys. J. Spec. Top. 224, 1349–1358 (2015).
Dunkel, J. et al. Fluid dynamics of bacterial turbulence. Phys. Rev. Lett. 110, 228102 (2013).
Wensink, H. H. et al. Meso-scale turbulence in living fluids. Proc. Natl Acad. Sci. USA 109, 14308–14313 (2012).
Slomka, J. & Dunkel, J. Geometry-dependent viscosity reduction in sheared active fluids. Phys. Rev. Fluids 2, 043102 (2017).
Slomka, J. & Dunkel, J. Spontaneous mirror-symmetry breaking induces inverse energy cascade in 3D active fluids. Proc. Natl Acad. Sci. USA 114, 2119–2124 (2017).
Tiribocchi, A., Wittkowski, R., Marenduzzo, D. & Cates, M. E. Active model H: scalar active matter in a momentum-conserving fluid. Phys. Rev. Lett. 115, 188302 (2015).
Cates, M. E. Active field theories. Preprint at arXiv https://arxiv.org/abs/1904.01330 (2019).
Mogilner, A. & Oster, G. Cell motility driven by actin polymerization. Biophys. J. 71, 3030–3045 (1996).
Dogterom, M., Kerssemakers, J. W., Romet-Lemonne, G. & Janson, M. E. Force generation by dynamic microtubules. Curr. Opin. Cell Biol. 17, 67–74 (2005).
Mogilner, A. Mathematics of cell motility: have we got its number? J. Math. Biol. 58, 105 (2008).
Erlenkämper, C. & Kruse, K. Uncorrelated changes of subunit stability can generate length-dependent disassembly of treadmilling filaments. Phys. Biol. 6, 046016 (2009).
Howard, J. & Kruse, K. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer Associates, 2001).
Nielsen, S. O., Bulo, R. E., Moore, P. B. & Ensing, B. Recent progress in adaptive multiscale molecular dynamics simulations of soft matter. Phys. Chem. Chem. Phys. 12, 12401–12414 (2010).
Ekimoto, T. & Ikeguchi, M. Multiscale molecular dynamics simulations of rotary motor proteins. Biophys. Rev. 10, 605–615 (2018).
Kolomeisky, A. B. & Fisher, M. E. Molecular motors: a theorist’s perspective. Annu. Rev. Phys. Chem. 58, 675–695 (2007).
Chowdhury, D. Stochastic mechano-chemical kinetics of molecular motors: a multidisciplinary enterprise from a physicist’s perspective. Phys. Rep. 529, 1–197 (2013). Physicist’s view on molecular motors.
Klumpp, S. & Lipowsky, R. Cooperative cargo transport by several molecular motors. Proc. Natl Acad. Sci. USA 102, 17284–17289 (2005).
Appert-Rolland, C., Ebbinghaus, M. & Santen, L. Intracellular transport driven by cytoskeletal motors: general mechanisms and defects. Phys. Rep. 593, 1–59 (2015).
Bausch, A. R. & Kroy, K. A bottom-up approach to cell mechanics. Nat. Phys. 2, 231–238 (2006).
Huber, F. et al. Emergent complexity of the cytoskeleton: from single filaments to tissue. Adv. Phys. 62, 1–112 (2013).
Broedersz, C. P. & MacKintosh, F. C. Modeling semiflexible polymer networks. Rev. Mod. Phys. 86, 995–1036 (2014).
Mohapatra, L., Goode, B. L., Jelenkovic, P., Phillips, R. & Kondev, J. Design principles of length control of cytoskeletal structures. Annu. Rev. Biophys. 45, 85–116 (2016).
Mogilner, A. & Craig, E. Towards a quantitative understanding of mitotic spindle assembly and mechanics. J. Cell Sci. 123, 3435–3445 (2010).
Pavin, N. & Tolić, I. M. Self-organization and forces in the mitotic spindle. Annu. Rev. Biophys. 45, 279–298 (2016).
Broedersz, C. P. & MacKintosh, F. C. Molecular motors stiffen non-affine semiflexible polymer networks. Soft Matter 7, 3186–3191 (2011).
Ronceray, P., Broedersz, C. P. & Lenz, M. Fiber networks amplify active stress. Proc. Natl Acad. Sci. USA 113, 2827–2832 (2016).
Jülicher, F., Kruse, K., Prost, J. & Joanny, J.-F. Active behavior of the cytoskeleton. Phys. Rep. 449, 3–28 (2007).
Joanny, J. F. & Prost, J. Active gels as a description of the actin–myosin cytoskeleton. HFSP J. 3, 94–104 (2009).
Nedelec, F. & Foethke, D. Collective langevin dynamics of flexible cytoskeletal fibers. New J. Phys. 9, 427–427 (2007).
Jilkine, A. & Edelstein-Keshet, L. A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput. Biol. 7, e1001121 (2011).
Holmes, W. R. & Edelstein-Keshet, L. A comparison of computational models for eukaryotic cell shape and motility. PLoS Comput. Biol. 8, e1002793 (2012).
Danuser, G., Allard, J. & Mogilner, A. Mathematical modeling of eukaryotic cell migration: insights beyond experiments. Annu. Rev. Cell Dev. Biol. 29, 501–528 (2013). Comprehensive overview of mathematical modelling of cell migration.
te Boekhorst, V., Preziosi, L. & Friedl, P. Plasticity of cell migration in vivo and in silico. Annu. Rev. Cell Dev. Biol. 32, 491–526 (2016).
Doubrovinski, K. & Kruse, K. Cell motility resulting from spontaneous polymerization waves. Phys. Rev. Lett. 107, 258103 (2011).
Wolgemuth, C. W., Stajic, J. & Mogilner, A. Redundant mechanisms for stable cell locomotion revealed by minimal models. Biophys. J. 101, 545–553 (2011).
Ziebert, F., Swaminathan, S. & Aranson, I. S. Model for self-polarization and motility of keratocyte fragments. J. R. Soc. Interface 9, 1084–1092 (2012).
Ziebert, F. & Aranson, I. S. Computational approaches to substrate-based cell motility. NPJ Comput. Mater. 2, 16019 (2016).
Linsmeier, I. et al. Disordered actomyosin networks are sufficient to produce cooperative and telescopic contractility. Nat. Commun. 7, 12615 (2016).
Singer-Loginova, I. & Singer, H. M. The phase field technique for modeling multiphase materials. Rep. Prog. Phys. 71, 106501 (2008).
Nonomura, M. Study on multicellular systems using a phase field model. PLoS One 7, 1–9 (2012).
Camley, B. A. et al. Polarity mechanisms such as contact inhibition of locomotion regulate persistent rotational motion of mammalian cells on micropatterns. Proc. Natl Acad. Sci. USA 111, 14770–14775 (2014).
Najem, S. & Grant, M. Phase-field model for collective cell migration. Phys. Rev. E 93, 052405 (2016).
Camley, B. A. & Rappel, W.-J. Physical models of collective cell motility: from cell to tissue. J. Phys. D 50, 113002 (2017). Overview of physical models describing collective motion of cells and tissues.
Mueller, R., Yeomans, J. M. & Doostmohammadi, A. Emergence of active nematic behavior in monolayers of isotropic cells. Phys. Rev. Lett. 122, 048004 (2019).
Wenzel, D., Praetorius, S. & Voigt, A. Topological and geometrical quantities in active cellular structures. J. Chem. Phys. 150, 164108 (2019).
Abaurrea-Velasco, C., Ghahnaviyeh, S. D., Pishkenari, H. N., Auth, T. & Gompper, G. Complex self-propelled rings: a minimal model for cell motility. Soft Matter 13, 5865–5876 (2017).
Abaurrea-Velasco, C., Auth, T. & Gompper, G. Vesicles with internal active filaments: self-organized propulsion controls shape, motility, and dynamical response. New J. Phys. https://doi.org/10.1088/1367-2630/ab5c70 (2019).
Preziosi, L., Ambrosi, D. & Verdier, C. An elasto-visco-plastic model of cell aggregates. J. Theor. Biol. 262, 35–47 (2010).
Rodriguez, E. K., Hoger, A. & McCulloch, A. D. Stress-dependent finite growth in soft elastic tissues. J. Biomech. 27, 455–467 (1994).
Drasdo, D. & Höhme, S. A single-cell-based model of tumor growth in vitro: monolayers and spheroids. Phys. Biol. 2, 133–147 (2005).
Basan, M., Prost, J., Joanny, J.-F. & Elgeti, J. Dissipative particle dynamics simulations for biological tissues: rheology and competition. Phys. Biol. 8, 026014 (2011).
Malmi-Kakkada, A. N., Li, X., Samanta, H. S., Sinha, S. & Thirumalai, D. Cell growth rate dictates the onset of glass to fluidlike transition and long time superdiffusion in an evolving cell colony. Phys. Rev. X 8, 021025 (2018).
Matoz-Fernandez, D. A., Martens, K., Sknepnek, R., Barrat, J. L. & Henkes, S. Cell division and death inhibit glassy behaviour of confluent tissues. Soft Matter 13, 3205–3212 (2017).
Graner, F. & Glazier, J. A. Simulation of biological cell sorting using a two-dimensional extended potts model. Phys. Rev. Lett. 69, 2013–2016 (1992).
Glazier, J. A. & Graner, F. Simulation of the differential adhesion driven rearrangement of biological cells. Phys. Rev. E 47, 2128–2154 (1993).
Chen, N., Glazier, J. A., Izaguirre, J. A. & Alber, M. S. A parallel implementation of the cellular Potts model for simulation of cell-based morphogenesis. Comput. Phys. Commun. 176, 670–681 (2007).
Maree, A. F. M. & Hogeweg, P. How amoeboids self-organize into a fruiting body: multicellular coordination in dictyostelium discoideum. Proc. Natl Acad. Sci. USA 98, 3879–3883 (2001).
Merks, R. M. H., Perryn, E. D., Shirinifard, A. & Glazier, J. A. Contact-inhibited chemotaxis in de novo and sprouting blood-vessel growth. PLoS Comp. Biol. 4, e1000163 (2008).
Maree, A. F. M., Jilkine, A., Dawes, A., Grieneisen, V. A. & Edelstein-Keshet, L. Polarization and movement of keratocytes: a multiscale modelling approach. Bull. Math. Biol. 68, 1169–1211 (2006).
Albert, P. J. & Schwarz, U. S. Dynamics of cell shape and forces on micropatterned substrates predicted by a cellular potts model. Biophys. J. 106, 2340–2352 (2014).
Segerer, F. J., Thöroff, F., PieraAlberola, A., Frey, E. & Rädler, J. O. Emergence and persistence of collective cell migration on small circular micropatterns. Phys. Rev. Lett. 114, 228102 (2015).
Hufnagel, L., Teleman, A. A., Rouault, H., Cohen, S. M. & Shraiman, B. I. On the mechanism of wing size determination in fly development. Proc. Natl Acad. Sci. USA 104, 3835–3840 (2007).
Fletcher, A. G., Osterfield, M., Baker, R. E. & Shvartsman, S. Y. Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291–2304 (2014).
Sussman, D. M., Schwarz, J. M., Marchetti, M. C. & Manning, M. L. Soft yet sharp interfaces in a vertex model of confluent tissue. Phys. Rev. Lett. 120, 058001 (2018).
Barton, D. L., Henkes, S., Weijer, C. J. & Sknepnek, R. Active vertex model for cell-resolution description of epithelial tissue mechanics. PLoS Comput. Biol. 13, e1005569 (2017).
Chiang, M. & Marenduzzo, D. Glass transitions in the cellular Potts model. Europhys. Lett. 116, 28009 (2016).
Bi, D., Yang, X., Marchetti, M. C. & Manning, M. L. Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X 6, 021011 (2016).
Oswald, L., Grosser, S., Smith, D. M. & Käs, J. A. Jamming transitions in cancer. J. Phys. D 50, 483001 (2017). Review of tissue dynamics and liquid-like versus solid-like behaviour.
Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues 2nd edn (Springer, 2010).
Dunlop, J. W. C., Fischer, F. D., Gamsjäger, E. & Fratzl, P. A theoretical model for tissue growth in confined geometries. J. Mech. Phys. Solids 58, 1073–1087 (2010).
Ambrosi, D., Preziosi, L. & Vitale, G. The interplay between stress and growth in solid tumors. Mech. Res. Commun. 42, 87–91 (2012).
Shraiman, B. I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005).
Byrne, H. & Preziosi, L. Modelling solid tumour growth using the theory of mixtures. Math. Med. Biol. 20, 341–366 (2003).
Tracqui, P. Biophysical models of tumour growth. Rep. Prog. Phys. 72, 056701 (2009).
Rieger, H. & Welter, M. Integrative models of vascular remodeling during tumor growth. WIREs Syst. Biol. Med. 7, 113–129 (2015).
Fredrich, T., Rieger, H., Chignola, R. & Milotti, E. Fine-grained simulations of the microenvironment of vascularized tumours. Sci. Rep. 9, 11698 (2019).
Romanczuk, P., Couzin, I. D. & Schimansky-Geier, L. Collective motion due to individual escape and pursuit response. Phys. Rev. Lett. 102, 010602 (2009).
Simpson, S. J., Sword, G. A., Lorch, P. D. & Couzin, I. D. Cannibal crickets on a forced march for protein and salt. Proc. Natl Acad. Sci. USA 103, 4152–4156 (2006).
Agudo-Canalejo, J. & Golestanian, R. Active phase separation in mixtures of chemically interacting particles. Phys. Rev. Lett. 123, 018101 (2019).
Pearce, D. J. G., Miller, A. M., Rowlands, G. & Turner, M. S. Role of projection in the control of bird flocks. Proc. Natl Acad. Sci. USA 111, 10422–10426 (2014).
Lavergne, F. A., Wendehenne, H., Bäuerle, T. & Bechinger, C. Group formation and cohesion of active particles with visual perception-dependent motility. Science 364, 70–74 (2019).
Ballerini, M. et al. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237 (2008).
Bajec, I. L. & Heppner, F. H. Organized flight in birds. Animal Behav. 78, 777–789 (2009).
Mijalkov, M., McDaniel, A., Wehr, J. & Volpe, G. Engineering sensorial delay to control phototaxis and emergent collective behaviors. Phys. Rev. X 6, 011008 (2016).
Charlesworth, H. J. & Turner, M. S. Intrinsically motivated collective motion. Proc. Natl Acad. Sci. USA 116, 15362–15367 (2019).
Khadka, U., Holubec, V., Yang, H. & Cichos, F. Active particles bound by information flows. Nat. Commun. 9, 3864 (2018).
Mann, R. P. & Garnett, R. The entropic basis of collective behaviour. J. R. Soc. Interface 12, 20150037 (2015).
Ward, A. J. W., Sumpter, D. J. T., Couzin, I. D., Hart, P. J. B. & Krause, J. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 (2008).
Abaurrea Velasco, C., Abkenar, M., Gompper, G. & Auth, T. Collective behavior of self-propelled rods with quorum sensing. Phys. Rev. E 98, 022605 (2018).
Castellano, C., Fortunato, S. & Loreto, V. Statistical physics of social dynamics. Rev. Mod. Phys. 81, 591–646 (2009).
King, A. J., Douglas, C. M., Huchard, E., Isaac, N. J. & Cowlishaw, G. Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838 (2008).
Couzin, I. D., Krause, J., Franks, N. R. & Levin, S. A. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (2005).
Freeman, R. & Biro, D. Modelling group navigation: dominance and democracy in homing pigeons. J. Navigat. 62, 33–40 (2009).
Bäuerle, T., Fischer, A., Speck, T. & Bechinger, C. Self-organization of active particles by quorum sensing rules. Nat. Commun. 9, 3232 (2018).
Moussaïd, M., Helbing, D. & Theraulaz, G. How simple rules determine pedestrian behavior and crowd disasters. Proc. Natl Acad. Sci. USA 108, 6884–6888 (2011).
Faria, J. J., Dyer, J. R., Tosh, C. R. & Krause, J. Leadership and social information use in human crowds. Animal Behav. 79, 895–901 (2010).
Bain, N. & Bartolo, D. Dynamic response and hydrodynamics of polarized crowds. Science 363, 46–49 (2019).
Kim, M.-C., Silberberg, Y. R., Abeyaratne, R., Kamm, R. D. & Asada, H. H. Computational modeling of three-dimensional ECM-rigidity sensing to guide directed cell migration. Proc. Natl Acad. Sci. USA 115, E390–E399 (2018).
Paluch, E. K. & Raz, E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 25, 582–590 (2013).
Tozluoglu, M. et al. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 15, 751–762 (2013).
Moure, A. & Gomez, H. Three-dimensional simulation of obstacle-mediated chemotaxis. Biomech. Model. Mechanobiol. 17, 1243–1268 (2018).
Besser, A. & Schwarz, U. S. Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction. New J. Phys. 9, 425–425 (2007).
Nishikawa, M., Naganathan, S. R., Jülicher, F. & Grill, S. W. Controlling contractile instabilities in the actomyosin cortex. eLife 6, e19595 (2017).
Gross, P. et al. Guiding self-organized pattern formation in cell polarity establishment. Nat. Phys. 15, 293–300 (2019).
Bratanov, V., Jenko, F. & Frey, E. New class of turbulence in active fluids. Proc. Natl Acad. Sci. USA 112, 15048–15053 (2015).
Weber, C. A. et al. Long-range ordering of vibrated polar disks. Phys. Rev. Lett. 110, 208001 (2013).
Acknowledgements
M.R.S., A.W. and H.R. acknowledge support by the Deutsche Forschungsgemeinschaft (DFG) within SFB 1027 (A3, A7). R.G.W. and G.G. acknowledge funding by DFG within the priority programme SPP 1726 “Microswimmers — from Single Particle Motion to Collective Behaviour”.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of manuscript preparation, revision and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shaebani, M.R., Wysocki, A., Winkler, R.G. et al. Computational models for active matter. Nat Rev Phys 2, 181–199 (2020). https://doi.org/10.1038/s42254-020-0152-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42254-020-0152-1
This article is cited by
-
Emergent memory from tapping collisions in active granular matter
Communications Physics (2024)
-
Non-Hermitian non-equipartition theory for trapped particles
Nature Communications (2024)
-
Complex motion of steerable vesicular robots filled with active colloidal rods
Scientific Reports (2023)
-
Motional consensus of self-propelled particles
Scientific Reports (2023)
-
The role of particle shape in computational modelling of granular matter
Nature Reviews Physics (2023)