Abstract and Introduction
Abstract
With the comprehensive study and complete sequencing of the Haemophilus influenzae genome in 1995 came the term 'genomics' and the beginning of the 'omics' era. Since this time, several analogous fields, such as transcriptomics and proteomics, have emerged. While growth and advancement in these fields have increased understanding of microbial virulence, the study of bacterial glycomes is still in its infancy and little is known concerning their role in host–pathogen interactions. Bacterial glycomics is challenging owing to the diversity of glyco-conjugate molecules, vast array of unusual sugars and limited number of analytical approaches available. However, recent advances in glycomics technologies offer the potential for exploration and characterization of both the structures and functions of components of bacterial glycomes in a systematic manner. Such characterization is a prerequisite for discerning the role of bacterial glycans in the interaction between host defences and bacterial virulence factors.
Introduction
Carbohydrates constitute the most structurally diverse class of natural products and can serve many functions in cells and organisms.[1] In addition to their role in energy metabolism, carbohydrates are also found attached to proteins and lipids, or as loosely associated polysaccharides on the cell surface. Glycobiology encompasses the determination of the structure and function of complex carbohydrates (glycans). More recently, there has been a shift within glycobiology towards large-scale systematic studies of the entire complement of glycan structures within a given cell or organism, refered to as the 'glycome'. Glycomics, therefore, is the study of the significance of glycoconjugate assembly and expression in biological systems. Since glycosylation involves a series of metabolic events, some of the concepts in lipidomics[2] and metabolomics[3] are common to glycomics. As a field, glycomics has lagged behind genomics and proteomics, owing to the inherent difficulties in the isolation of glycans, the subsequent analysis of their structure and function and the limited number of different analytical techniques available for use in their study.
Surface polysaccharides represent the predominant structures on all bacterial cell surfaces, and they are often important players in the interactions between pathogens, their hosts and the environment. These structures can be involved in the maintenance of surface charge, phase variation and serum resistance. In bacteria, the role of these glycan moieties in symbiosis, pathogenesis, biofilm formation, cell–cell interactions and evasion of the immune response[4–7] is just beginning to be understood. In Gram-negative bacteria, the vast majority of carbohydrate moieties are found in lipopolysaccharides (LPS)/lipooligosaccharides (LOS) or capsular polysaccharide (CPS). There are an increasing number of reports describing the modification of bacterial proteins with N- or O-linked glycan moieties (Figure 1) (reviewed in [8,9]).
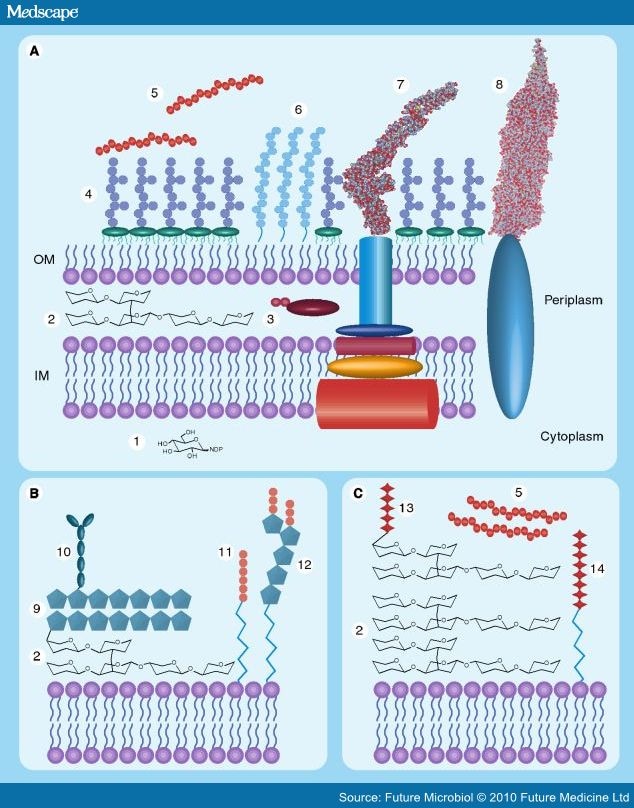
Figure 1.
Microbial glycome diversity. (A) The Gram-negative cell showing the various components that constitute the glycome: (1) nucleotide-linked sugar precursors; (2) peptidoglycan; (3) N-linked glycoproteins; (4) lipopolysaccharide/oligosaccharide; (5) extracellular polysaccharide; (6) capsular polysaccharide; (7) O-linked protein glycosylation, FliC, Salmonella enterica (PDB: 1UCU); (8) pilin Neisseria gonorhoeae (PDB: 2HIL). (B) The mycobacterial glycome in addition to possessing nucleotide-linked sugar precursors and peptidoglycan the cell surface contains: (9) arabinogalactan, (10) mycolic acids, (11) lipomannan and (12) lipoarabinomannan. (C) The Gram-positive cell produces many of the same glycoconjugates as the Gram-negative cell, such as capsular polysaccharide, extracellular polysaccharide and a thicker peptidoglycan layer but, in addition, produces the cell surface components (13) teichoic and (14) lipoteichoic acids.
IM: Inner membrane; OM: Outer membrane.
One of the main challenges in the study of glycomes is the complexity of carbohydrate structures. In mammals, carbohydrates are assembled from a group of approximately ten common monosaccharides. By contrast, the absolute number of potential monosaccharide building blocks in bacteria is unknown. Bacteria can synthesize pentose and heptose sugars not commonly found in mammals, and this diverse array of glycans is proving to be an analytical challenge. In addition, carbohydrate complexity increases as monosaccharides are assembled into linear and branched polymers based on the regiochemistry and stereochemistry (α or β) of the anomeric carbon in the glycosidic bond. For example, it is estimated that, for a hexasaccharide, all possible oligosaccharide isomers would yield approximately 1012 structures.[10] In addition to the monosaccharide and linkage diversity, a number of modifications to the monosaccharide subunits can occur, including methylation, sulfation, phosphorylation and, in some bacteria, the addition of amino acids.[11,12] Finally, the method of display of the glycan, by attachment to lipids or via N- and O-linkage to proteins, adds further complexity to the glycome. This complexity is compounded by the nontemplate-driven nature of glycan biosynthesis. Taking all these factors into consideration, the analytical challenge to dissect these structures, and assign biological function, is substantial.
In this review we provide an overview of bacterial glycomes and the analytical techniques that are employed in their study. Owing to the diversity in this field, it is not feasible to discuss all the contributions made to bacterial glycomics; therefore, this review will focus on surveying the recent literature and describing select techniques that can be applied to the analysis of the bacterial glycome. We then focus upon how this work has specifically contributed to our understanding of the role of the bacterial glycome in the host–pathogen interactions of Campylobacter jejuni.
Future Microbiol. 2010;5(2):267-288. © 2010 Future Medicine Ltd.
Cite this: Never Take Candy from a Stranger: The Role of the Bacterial Glycome in Host–Pathogen Interactions - Medscape - Feb 01, 2010.






Comments