Pulmonary Administration of Peptides & Therapeutic Proteins
Lungs Anatomical & Physiological Characteristics
Through pulmonary administration it is possible to access the systemic circulation by an epithelium of low thickness (0.1–0.5 µm in alveolar region)[3,18] and high surface area for absorption (75–150 m2).[19–21] In addition, it has intra- and extracellular enzyme activity and drug efflux systems below the gastrointestinal tract and an extensive blood supply. This is a noninvasive route of administration, which allows local and systemic therapies with a rapid onset of action and avoids the first-pass effect.[8,18,22]
Moreover, the bioavailability of proteins and peptides administered via pulmonary administration is 10–200-times greater when compared with other noninvasive routes, owing to a more permeable epithelium and a reduced volume of fluid that allows high concentrations of drug near the bloodstream (Table 1).[23,24]
All these features make the inhalation technique a promising alternative to parenteral administration of various proteins and peptides.[25]
Pulmonary Clearance & Absorption
After administration, peptides and proteins will undergo lung deposition and be subject to the existing clearance mechanisms in the respiratory system.
There are several proposed mechanisms for the clearance of proteins in the lungs and they are responsible for reducing the bioavailability of these after inhalation. However, these are complex and not yet fully clarified.[26] Include mucociliar escalator, phagocytosis by macrophages, intracellular catabolism and permeation through the alveolar epithelium.[27] After being phagocytosed by macrophages, proteins can be transported by the alveolar surface, undergo translocation to the lymphatic system or degradation by intracellular enzymatic lysosomal system.[18]
Studies show that the rate of lung protein clearance did not change significantly in the presence or absence of an endotracheal tube, suggesting a reduced role of the mucociliar escalator in protein clearance.[28] In addition, they demonstrate that only small amounts of protein are found in macrophages.[28] Despite the fact that the phagocytic capacity of macrophages is higher than the endocytic capacity of pneumocytes, these will not begin to play an important role in clearance of proteins until 48 h after exposure.[27,28] In addition, several large proteins reach the circulation in intact form after instillation, suggesting a lower impact of the first three mechanisms in lung protein clearance.[26,27] In the case of proteins that undergo a high catabolism, the use of enzyme inhibitors such as bacitracin, chymostatin, leupeptin or nafmostato mesylate, reduce proteolysis and thereby increase their bioavailability.[17]
As previously mentioned, it appears that the alveolar epithelium is considered the main barrier in lung protein clearance.[26] This is composed of polarized cells permeable to water, gases and lipophilic molecules. However, the permeation of hydrophilic substances of large molecular size such as proteins, and of ionic species, is limited,[17] with the molecular weight cutoff of tight junctions for alveolar type I cells at 0.6 nm.[29]
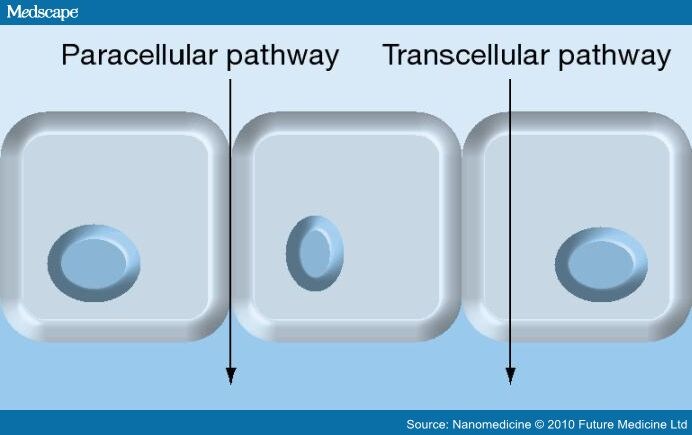
The permeation of molecules through biological membranes is a complex phenomenon. Simultaneous pathways may be involved in the transcellular and paracellular pathway (Figure 1).[30]
Figure 1.
Molecule absorption routes.
Despite the existing transportation systems, in the lung epithelium are poorly characterized and it is known that the absorption rate of various proteins across the epithelium is size-dependent.[26] Several authors have demonstrated the existence of an inverse relationship between the molecular weight of macromolecules and their absorption rates.[31–33] However, this relationship is particularly true for proteins that undergo absorption by paracellular mechanism. The temperature also interferes with the absorption of protein and there is a direct relationship between the two.[26,27]
Peptides and proteins of low molecular weight cross the alveolar epithelium mainly by the paracellular pathway. This also prevails when there is epithelial injury such as edema or inflammation. In the permeation of proteins of higher size, the transcellular route, especially endocytosis, appears to be more involved.[26,34] Peptides and peptidomimetics drugs can be absorbed by active transport using the high-affinity peptide transporter (PEPT)2 existing in alveolar type II and in capillary endothelium.[21,35] The presence of caveolin in alveolar type I and endothelial cells of lung and clathrin-coated vesicles in alveolar type I and II suggests the possible involvement of such pathways in the absorption of proteins.[26]
To increase the absorption of proteins it is possible to use permeation enhancers as surfactants, bile salts and cyclodextrins.[17]
Limitations of Pulmonary Administration
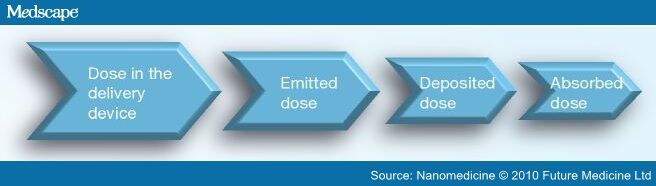
The main limitation of pulmonary administration of drugs relates to the reproducibility of the dose. Between delivery and absorption in the lungs, the drug undergoes consecutive losses, so the absorbed dose is usually below the dose present in the delivery device (Figure 2).
Figure 2.
Variation of the drug dose from the delivery device to absorption.
The deposition of particles at the lower respiratory tract is a complex phenomenon and its efficiency depends on several factors including the type of formulation, delivery device used and their capacity to produce the aerosol. It also depends on physiological factors such as humidity and geometry of the airways, respiratory capacity (inspiratory flow rate, breathing frequency and tidal volume) and the inhalation technique used by the patient, as well as factors inherent to the particles such as the mean diameter, surface and shape, density and their aerodynamic properties.[20,36–38] The delivery efficiency of particles is reduced in patients with lower respiratory capacity, for example, children, elderly persons or adults with certain disease conditions that compromises their lung function, resulting in nonreproducible pharmacokinetic and pharmcodynamic responses.[29]
The delivery devices play an important role in the efficiency of alveolar deposition. The most common are nebulizers, metered dose inhalers (MDI) and dry powder inhalers (DPI), which were designed for managing small molecules especially for local delivery and not for biopharmaceuticals. For this reason, in recent years the development of new and improved devices for administration of peptides and proteins has been intensified. Examples of such devices are the AERx® from Aradigm (CA, USA), Respimat® from Boehringer Ingelheim (Ingelheim, Germany) or AeroDose® from Aerogen Inc. (Galway, Ireland).[17] Unfortunately, there is no device producing only particles within the size limits appropriate to the lung deposition, which results in a very low rate of dose emitted.[36]
An issue of relevance arises from the fact that a proportion of patients (over 50%) use the delivery device improperly, leading to nonreproducibility.[37] Thus, an intensive education of patients by healthcare professionals leads to an increase on the effectiveness of the treatment.[36]
State of the Art in Peptide & Protein Inhalation
In 1993 the US FDA approved the first protein administered via inhalation, the recombinant human desoxiribonuclease, also known as Dornase α (Pulmozyme®, Genentech Inc., CA, USA), for the treatment of cystic fibrosis.[39] Currently, there are several peptides and proteins with therapeutic potential such as insulin, calcitonin, leuprolide or interferons for which pulmonary administration is under development and in clinical trials (Table 2).
Insulin Insulin is the most studied protein and there are many studies and formulations developed for administering insulin by pulmonary route. Such formulations are in various stages of development, with one approval for marketing by the FDA and European Medicines Agency (EMA) (Exubera®, Pfizer, NY, USA) (Table 3). Insulin is a polypeptide hormone with a molecular weight of approximately 6 kDa produced in β cells exist in pancreatic islets of Langerhans.[37,38] Its production has the function to maintain stable glucose levels during feeding and fasting, yet regulating lipid and protein metabolism. Actually, insulin is used in the treatment of diabetes mellitus and its first pulmonary administration in humans was reported in 1925 by Gänsslen.[29]
In general, patients who received inhaled insulin formulations demonstrate similar postprandial glycemic control and values of glycated hemoglobin (HbA1c), faster onset of action, lower weight gain, lower incidence and severity of hypoglycemia and greater satisfaction (higher comfort and convenience) compared with patients receiving subcutaneous injection of regular insulin.[40–43] However, Pfizer decided to withdraw Exubera from the market because it did not achieve the financial expectations. Because it is a short-term insulin, it is necessary to inject a long-acting insulin for overnight glycemic control.[44,45] Moreover, the bioavailability of insulin in this formulation was very low, 10–20% of subcutaneous, and the complex inhalation device increased costs of therapy and decreased the compliance and acceptance by physicians and patients.[2,7] Also for commercial reasons, clinical trials of the AERx and AIR were discontinued.[46] With regard to adverse effects and their frequency, these are similar to subcutaneous insulin with the exception of coughing, which diminishes with prolonged use, and dyspnea in some patients. There is also an increase of serum anti-insulin after pulmonary administration, which has not yet been associated with any clinically significant alterations and stabilizes after 6–12 weeks.[41,43]
Pfizer announced in a statement the occurrence of a greater number of cases of lung cancer in diabetic patients also smokers who used Exubera compared with other treatments for Diabetes. The lung cancer cases may be due to the vasodilator and growth-promoting capacity of insulin.[41] However, owing to the low number of cases is not possible to establish a causal relationship between the emergence of lung cancer and the use of Exubera.
Calcitonin Calcitonin is an endogenous polypeptide hormone with a molecular weight of 3.4 kDa, produced by the parafollicular cells of the thyroid gland.[47] It plays a crucial role in calcium homeostasis and bone remodeling by inhibition of bone resorption, promoting new bone formation, moving calcium from blood to bone and enhancing the rate of calcium deposition.[47–49] In fact, calcitonin is used intravenously or intranasally in the clinical treatment of bone metabolic disorders such as osteoporosis and Paget's disease and the treatment of hypercalcemia and bone metastasis.[47,48] Although the intranasal administration is a noninvasive route to deliver calcitonin, it presents a low and variable bioavailability (ranging from 0.3–30.6%) and some side effects such headache, dizziness, nausea or nasal secretions. Pulmonary administration could reduce or avoid these side effects and, generally, presents higher bioavailability of peptides and proteins than intrasnasal administration.[49] In one study, a dry power formulation for inhalation showed 66% of the bioactivity and 28% of the bioavailability of intramuscular calcitonin.[50] In another study, the bioavailability of a (Asu1,7)-eel calcitonin solution containing sodium glycocholate as absorption enhancer after intratracheal instillation was 52.9% compared with intravenous administration. The formulation did not cause serious damage or local irritation to the pulmonary epithelium suggesting its possible use in the administration of calcitonin through the lung.[51]
Cyclosporin A Cyclosporin A (CsA) is a hydrophobic cyclic peptide with high immunosuppressive activity that could be used in immunological diseases such as pulmonary chronic asthma, hypersensitivity, bronchiolitis, sarcoidosis[52] or the treatment of lung transplant rejection.[53] Pulmonary delivery of CsA seems to be a viable alternative to parenteral administration.[54] As a local administration, this will reduce the long-term nephrotoxicity of oral and parenteral CsA. Furthermore, it allows a higher concentration of CsA at the site of action.[55] However, the use of solvents such as ethanol and propylene glycol in conventional formulations for pulmonary administration can cause adverse effects.[55]
IL-2 IL-2, also known as aldesleukin, a cytokine of 15.5 kDa, is an immunomodulatory drug approved for the treatment of renal cell carcinoma and advanced melanoma, especially when they are metastatic in the lungs. It is also used in other metastatic cancers and diseases characterized by states of immunodeficiency.[56,57] Intravenous administration of IL-2 is associated with severe and dose-limiting side effects in the kidneys, cardiovascular system and liver, among others.[58] In recent years, alternative methods of administration, including inhalation, have been studied and are the subject of clinical trials, obtaining positive results, especially in reducing associated side effects and increasing the median survival of patients.[59,60] Since 2006, the EMA has granted orphan designation to an inhaled human IL-2 produced by Immunservice GmbH, for the treatment of renal cell carcinoma.
Nanomedicine. 2011;6(1):123-141. © 2011
Future Medicine Ltd.
Cite this: Nanocarriers for Pulmonary Administration of Peptides and Therapeutic Proteins - Medscape - Jan 01, 2011.







Comments