Abstract
We aimed to explore the relationship between GLP-1 receptor (GLP-1R) expression in adipose tissue (AT) and incretin secretion, glucose homeostasis and weight loss, in patients with morbid obesity and type 2 diabetes undergoing bariatric surgery. RNA was extracted from subcutaneous (SAT) and visceral (VAT) AT biopsies from 40 patients randomized to metabolic gastric bypass, sleeve gastrectomy or greater curvature plication. Biochemical parameters, fasting plasma insulin, glucagon and area under the curve (AUC) of GLP-1 following a standard meal test were determined before and 1 year after bariatric surgery. GLP-1R expression was higher in VAT than in SAT. GLP-1R expression in VAT correlated with weight (r = −0.453, p = 0.008), waist circumference (r = −0.494, p = 0.004), plasma insulin (r = −0.466, p = 0.007), and systolic blood pressure (BP) (r = −0.410, p = 0.018). At 1 year, GLP-1R expression in VAT was negatively associated with diastolic BP (r = −0.361, p = 0.039) and, following metabolic gastric bypass, with the increase of GLP-1 AUC, (R2 = 0.46, p = 0.038). Finally, GLP-1R in AT was similar independently of diabetes outcomes and was not associated with weight loss after surgery. Thus, GLP-1R expression in AT is of limited value to predict incretin response and does not play a role in metabolic outcomes after bariatric surgery.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 (GLP-1) is a potent stimulator of glucose-dependent insulin secretion, and also confers beneficial actions on gastric emptying and appetite regulation1,2,3. GLP-1 exerts its effects principally through its interaction with the G protein-coupled receptor GLP-1R, which in humans is broadly expressed in the central nervous system and in peripheral tissues such as pancreas, heart, kidney, gastrointestinal tract and adipose tissue (AT)4. However, the extrapancreatic mechanisms and actions of GLP-1, especially in AT, remain insufficiently understood5,6,7,8,9. Accumulated data from in vitro studies in rat and human cell lines have described both lipogenic and lipolytic activity for GLP-1 that seems to be dependent on its concentration5,6,7,8,10,11,12. Moreover, studies in murine 3T3-L1 preadipocytes indicated that GLP-1 promotes pre-adipocyte differentiation and inhibition of apoptosis13,14,15. Interestingly, GLP-1R has been recently implicated in energy metabolism by directly stimulating mitochondrial bioenergetics and brown AT remodeling16,17. Some authors have hypothesized that promotion of adipogenesis through activation of GLP-1R increases the capacity of visceral hypertrophic adipocytes to store lipids, thereby decreasing ectopic lipid accumulation and improving insulin resistance (IR)18. In humans, GLP-1R expression was increased in visceral AT (VAT) from morbid obese subjects with a high insulin-resistant profile as compared with insulin-sensitive lean, overweight, and low insulin-resistant obese groups8. Furthermore, high GLP-1R expression in VAT predicted a greater improvement in the systemic IR index after biliopancreatic diversion surgery8.
In view of the aforementioned observations and considering that VAT dysfunction has been classically linked to inflammation and IR19,20,21, then it does not seem unreasonable to predict an additional role for this incretin receptor in AT metabolic functions, which might influence the recovery of glucose homeostasis and IR after massive weight loss. Accordingly, the aim of the present study was to explore the relationship between GLP-1R expression in AT and incretin secretion, glucose homeostasis, metabolism and weight loss after bariatric surgery. To do this, we used a well-characterized cohort of patients with morbid obesity and type 2 diabetes mellitus (T2D) undergoing bariatric surgery in the setting of a randomized controlled trial (RCT).
Matherial and Methods
Study design and setting
This study was part of a prospective single centre, non-blinded RCT, including patients with morbid obesity and T2D undergoing bariatric surgery. The trial was registered on January 25, 2016 at www.controlled-trials.com (ISRCTN14104758). Patients were consecutively recruited from the diabetes outpatient clinic of Bellvitge University Hospital, Barcelona, Spain. The study was conducted according to the principles of the Declaration of Helsinki. All patients signed an informed consent and the protocol study (PI11/01960) was approved by the Clinical Research Ethics Committee of Bellvitge University Hospital (reference PR 143/11).
The inclusion criteria were age between 18 and 60 years, body mass index (BMI) 35–43 kg/m2, T2D on hypoglycemic agents alone, insulin, or both. The exclusion criteria were the following: type 1 diabetes or positivity for glutamic acid decarboxylase autoantibodies, secondary forms of diabetes, acute metabolic complications, liver disease, renal dysfunction or patients under anticoagulant treatment, previous bariatric surgery, congenital or acquired abnormalities of the digestive tract, pregnancy, nursing or desired pregnancy in the 12 months following inclusion, and corticoid use by oral or intravenous route for more than 14 consecutive days in the last three months.
Randomization
Patients were randomly assigned 1:1:1 to undergo metabolic Roux-en-Y gastric bypass (mRYGB), sleeve gastrectomy (SG) or greater curvature plication (GCP). The randomization process was performed by the statistics department at Bellvitge University Hospital. A computer random number generator with opaque sealed sequentially numbered envelopes with stratification according to baseline levels of HbA1c (greater or lower/ equal to 7%) was used.
Study protocol
In the mRYGB procedure, a 100-mL gastric pouch is created along with a 200-cm biliopancreatic limb and an alimentary limb of 100 cm. SG is a restrictive technique consisting of a gastric volume reduction of 75–80% by resecting the stomach over a 36-French catheter beginning 4 cm from the pylorus and ending at the angle of His. GCP is a reversible restrictive technique with no stomach resection, in which an invagination of the greater gastric curvature is performed with two running non-absorbable sutures, calibrated over a 36-French catheter. A physical examination with determination of anthropometrical parameters and a complete biochemical analysis was performed before bariatric surgery and at months 1, 3, 6, and 12 following the procedure.
Buse’s criteria were used to define T2D remission in each procedure at 1 year of follow-up: Complete remission = HbA1c < 6% and fasting glucose < 100 mg/dl (<5.6 mmol/l) with no diabetes drugs for 1 year; partial remission = HbA1c < 6.5% and fasting glucose < 100–125 mg/dl (5.6–6.9 mmol/l) with no diabetes drugs for 1 year22.
Anthropometric measurements
Body weight, height and waist circumference, and blood pressure were measured at every visit. Weight change after surgery was reported as total weight loss percentage (TWL%) [(preoperative weight − current weight)/(preoperative weight) × 100]. Body composition was analysed by dual-energy X-ray absorptiometry (Hologic QDR 4500; Hologic Inc., Waltham, MA) before and 12 months after surgery.
Standard meal test
The standard meal test (SMT) was performed before bariatric surgery and at months 1 and 12. The SMT consisted of 200 ml of a liquid meal (Edanec®) containing 54% carbohydrates, 16% proteins, and 30% lipids, equivalent to 1 kcal/ml. Incretin-related treatments were discontinued at least 1 month before the analysis; the remainder of diabetes treatments, excluding insulin, were withdrawn at least 1 week before the SMT. Blood was drawn immediately before and 15, 30, 60 and 120 minutes following the SMT for determination of GLP-1 concentration23. Fasting glucagon levels were determined before the SMT. There were no changes to methods after trial commencement.
Laboratory determinations
Glucose, cholesterol and triglycerides were determined using standard enzymatic methods. Plasma insulin was analysed by immunoassay (Coat-A-Count Insulin; Diagnostic Products Corp., Los Angeles, CA); inter- and intra-assay variability was 6.6–12.4% and 5.8–21.7%, respectively. GLP-1 was measured by radioimmunoassay (Millipore, Saint Charles, MO); inter and intra-assay variability was 10–23% and 22–38%, respectively. To obtain complete dipeptidyl peptidase IV inhibition, all samples were processed with a DPP-IV inhibitor (DPP4–010; Merck, Madrid, Spain). Glucagon was measured by enzyme immunoassay (Yanahaira Institute Inc., Awakura, Fujinomiya-shi Shizuoka, Japan).
Gene expression analysis
Total RNA was extracted from subcutaneous AT (SAT) and VAT biopsies obtained during surgery using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). One microgram of RNA was reverse transcribed with random primers using the reverse transcription system high capacity cDNA kit (Applied Biosystems, Foster City, CA). Quantitative GLP-1R expression was evaluated with Taqman low-density arrays (Hs00157705_m1; Applied Biosystems microfluidic cards) on a 7900HT Fast Real-Time PCR platform. The relative expression was measured using the 2−ddCt method. Peptidyl-prolylisomerase A (PPIA, Hs04194521_s1) was used for normalization of gene expression levels and calculation of dCt values due to its stability across tissues and samples24,25.
Outcomes
The primary outcome of the study was the predictive value of gut hormone dynamics (GLP-1, glucagon, peptide-YY [PYY] and ghrelin) on glucose metabolism improvement at 1 and 12 months after surgery for each procedure. The study had two main secondary outcomes. The first was to compare the rate of T2D remission in each procedure at 1 year of follow-up, defined by Buse’s criteria22. The results of the previous primary and secondary outcomes have been published26. The other secondary outcome was the determination of the role of GLP-1 R expression in AT in incretin response, weight loss, insulin sentivity improvement and diabetes remission after surgery. There were no changes to trial outcomes after commencement of the study.
Statistical analysis
Based on preliminary data, the study was designed to detect a 20% difference in GLP-1 secretion (measured by the area under the curve [AUC] after the SMT) before and 1 month after bariatric surgery, with a power of 80% and α risk of 0.05. At least 10 subjects per group were considered, but to account for the possibility of loss at follow-up 15 patients per group were finally assigned. Biochemical and hormonal parameters were compared between procedures by analysis of variance (ANOVA). Categorical variables were compared using the Chi-square test and quantitative variables using ANOVA or the Kruskal-Wallis test. GLP-1R mRNA expression data were log transformed for analysis. GLP-1 AUC was calculated by the trapezoidal method27. Bivariate (Pearson or Spearman) and multivariate regression analyses were employed to determine the associations of GLP-1R expression in SAT and VAT with glucose, lipid profile, IR and incretin concentration. Moreover, the association of GLP-1R expression with T2D remission, homeostasis model assessment (HOMA)-IR and HOMA-β, weight loss and incretin secretion at 1 year was also tested. As this was an exploratory study, we did not make adjustments for multiple comparisons as the use of more stringent criteria could increase the likelihood of missing clinically significant associations between variables. Statistical analysis was performed using R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A P-value < 0.05 was considered statistically significant.
Results
From May 2012 to February 2014, 45 morbidly obese patients with T2D, aged 49.4 ± 7.9 years, BMI 39.4 ± 1.9 kg/m2, initial HbA1c 7.7 ± 1.9%, were consecutively randomized to mRYGB, SG or GCP (n = 15 in each group). All patients completed the study with the exception of one patient allocated to the SG group who withdrew from the study after 10 months. Paired samples from SAT and VAT were obtained during surgery in 40 patients (n = 15 mRYGB, n = 13 SG and n = 12 GCP), and all patients completed the 12 months study period. Baseline clinical and biochemical characteristics of the 40 patients are listed in Table 1.
As a summary of previously published data26, TWL% was significantly greater in the mRYGB group than in SG and GCP groups at 12 months: −35.29 ± 8.17 vs. −27.26 ± 5.66 vs. −20.24 ± 7.49%, respectively, p < 0.05. At 1 year of follow-up, HbA1c was significantly lower in the mRYGB group than in the SG and GCP groups: 5.09 (0.62) vs. 6.24 (0.85) vs. 6.79 (1.39) %, respectively, p < 0.05. After surgery, the increase in the AUC for GLP-1was significantly higher in the mRYGB group than in SG and GCP groups: 87.3 [6.21;163] vs. 22.2 [2.40;32.9] vs. −0.28 [−23.94;40.5], respectively, p < 0.05. Short-term complete diabetes remission at 1 year was found in 80% of patients in the mRYGB group (n = 12), 53.8% in the SG group (n = 7) and 16.7% in the GCP group (n = 2), p < 0.001.
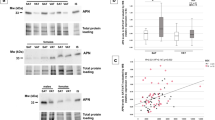
Analysis of GLP-1R expression in AT biopsies at surgery revealed a higher expression of the receptor in the visceral than in the subcutaneous depots (Fig. 1). Nonetheless, when we analysed GLP-1R expression in relation to patients’ baseline characteristics, VAT GLP-1R expression was inversely correlated with weight (r = −0.453, p = 0.008) and waist circumference (r = −0.494, p = 0.004), indicating that patients with greater weight and waist circumference had lower GLP-1R expression in this depot (Fig. 2). No association was found between GLP-1R expression in VAT and other anthropometrical parameters such as body fat or BMI. Regarding biochemical parameters, VAT GLP-1R expression was inversely correlated with basal plasma insulin (r = −0.466, p = 0.007), indicating that patients with higher insulin concentrations had lower GLP-1R expression. No differences in VAT GLP-1R expression were found with glucose metabolism parameters (glucose and HbA1c), HOMA-IR, HOMA-β or lipid profile. Similarly, there was no correlation between VAT GLP-1R expression and pre-surgical GLP-1 AUC in plasma or fasting glucagon concentrations. Regarding blood pressure, VAT GLP-1R expression was inversely correlated with systolic blood pressure (r = −0.410, p = 0.018) (Table 2). A multivariate regression analysis to account for confounders was performed finding that VAT GLP-1R expression remained inversely correlated with weight (R2 = 0.223, p = 0.049) after adjusting for gender, BMI, insulin and HbA1c. Analysis of GLP-1R expression in SAT showed no significant associations with anthropometrical, biochemical or hormonal parameters.
When we analysed AT GLP-1R expression using parameters at 12 months after surgery, we found an inverse association between GLP-1R expression in VAT and diastolic blood pressure at 12 months (r = −0.361, p = 0.039), however this correlation lost significance in the multivariate regression analysis after adjusting for gender, BMI, HbA1c and type of surgery. No other correlations were found between VAT or SAT GLP-1R expression in the bivariate and multiple regression analysis and glucose metabolism parameters or lipid profile at 12 months (Table 3), or with their changes after the intervention.
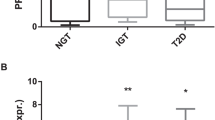
Regarding the association between GLP-1R expression in AT and GLP-1 concentration, only those patients undergoing mRYGB, characterized by a greater metabolic improvement and higher incretin response, showed a negative relationship between GLP-1R expression in VAT and the increase of GLP-1 AUC at 1 year after surgery (r = −0.670, p = 0.015). In multiple regression analysis, VAT GLP-1R expression after mRYGB remained inversely associated with the increase of GLP-1 AUC at 1 year after surgery, after adjusting for changes in weight, BMI and HbA1c (R2 for the model 0.46, p = 0.038). However, GLP-1R expression in AT did not predict metabolic outcomes. Accordingly, no correlations were observed between GLP-1R expression in AT and glucose, HbA1c or insulin sensitivity improvement calculated by HOMA-IR or HOMA-β after the intervention. Moreover, GLP-1R expression at VAT and SAT did not differ between patients remaining with T2D compared with those under partial or complete T2D remission after the bariatric procedure: 0.29 (0.04;0.82) vs. 1.05 (0.22;2.10) vs. 0.28 (0.08;2.18), p = 0.779 and 0.02 (0.0009;0.007) vs. 0.03 (0.0026;0.32) vs. 0.17 (0.01;0.37), p = 0.278, respectively (Fig. 3). Finally, GLP-1R expression in AT was not associated with weight response after surgery.
Discussion
In the present study, we evaluated the clinical significance of GLP-1R expression in AT in relation to plasma incretin concentration, glucose homeostasis, insulin sensitivity improvement and weight outcome in patients with T2D undergoing bariatric surgery in an RCT. In subjects randomized to mRYGB, basal GLP-1R expression in VAT was an inverse determinant of the increase in incretin concentration after surgery. However, GLP-1R expression in AT was not associated with diabetes remission or weight loss after surgery.
GLP-1R is broadly expressed in many tissues beyond the pancreas, including lung, brain, stomach, heart, kidney, endothelium, bone and also AT4. The physiological actions of GLP-1R have been well documented over the last decade, especially after the extensive clinical applications of GLP-1 mimetics for diabetes, such as GLP-1R agonists and DPP-4 inhibitors1,2. However, the significance of GLP-1R in AT is not fully understood and remains contentious. Data from in vitro studies indicate that GLP-1 has a role in adipogenesis5,6,7,8,9,10,11,12,13,14,15. Murine 3T3-L1 adipocyte precursors treated with GLP-1 and exendin-4 (a GLP-1R agonist) showed increased differentiation14, favouring intracellular lipid accumulation and insulin-mediated glucose uptake through up-regulation of Glut-4 expression and phosphorylation of the insulin receptor in adipocytes28. While the evidence is less established in humans, in mature adipocytes GLP-1 treatment induced a significant lipolytic effect in a dose-dependent manner8,12. More recently, treatments with GLP-1 analogues have been associated with increased proliferation and inhibition of apoptosis in human adipose-derived stromal cells29, and also with stimulation of mitochondrial bioenergetics in AT16,17.
In addition to mature adipocytes, AT also contain various cell populations in the stromal vascular fraction (SVF), including mesenchymal stem cells (MSCs), preadipocytes, fibroblasts, vascular endothelial cells, and a range of immune cells such as AT macrophages. GLP-1R has been found expressed in mature adipocytes and MSCs, but the expression in the rest of SVF components remain elusive8. The GLP-1R agonist exendin-4 is able to inhibit LPS-induced TNF-α expression in macrophages, which is an indirect evidence of the anti-inflammatory role of GLP-1 in macrophages30. Also, inhibition of nuclear factor-kappa B (NF-κB) activation of macrophage population in AT in the presence of GLP-1R analogs has been reported31. Furthermore, endothelial vascular cells are sensitive to the GLP1-R analogs, with a net anti-inflammatory effect through different molecular mechanisms driven by the activation of adenosine monophosphate-activated protein kinase (AMPK)32. Altogether, previous data would suggest that GLP-1R expression within AT is due to the contribution of its two major components, mature adipocytes and the cellular components of the SVF, with a predominant anti-inflammatory effect in the last location.
GLP-1R expression has been described in AT of lean subjects, with higher expression in visceral than in subcutaneous depots, and with lower expression than in insulin-resistant morbidly obese subjects8. In line with a previous report, we observed higher expression of GLP-1R in VAT than in SAT compartments8. Consistent with this, a recent study identified GLP-1R expression in epicardial AT, which represents the visceral fat equivalent of the heart33. Interestingly, an excess of epicardial fat has been implicated in IR and cardiovascular diseases, exerting its atherogenic action through the paracrine secretion of pro-inflammatory cytokines34. Similarly, an increase in abdominal VAT is associated with an increased metabolic, cardiovascular risk and greater mortality prediction capacity20,21,35. Many of these deleterious events are mediated, in part, by inflammatory mechanisms originating in the AT depots. Indeed, recent data suggest that GLP-1-based therapies may have anti-inflammatory effects in several organs including AT, by reducing the production of pro-inflammatory cytokines and infiltration of immune cells31,36. Therefore, improving the knowledge of the regulatory dynamics of the GLP1/GLP-1R axis in AT might have relevance for the treatment of local and systemic inflammatory events in obesity and T2D.
In patients with morbid obesity without T2D, the expression of GLP-1R in VAT has been described to be positively associated with the degree of IR and with the greatest insulin sensitivity improvement after biliopancreatic diversion surgery8. While our results in patients with T2D support this association, our findings revealed a negative correlation between insulin levels and GLP-1R expression in VAT. This is consistent with the negative relationship observed between weight, waist circumference and blood pressure and GLP-1R expression in this depot; thus, the greater the metabolic disturbances the lower the GLP-1R expression in AT. It should be highlighted that our cohort comprised patients with morbid obesity and T2D, and this may result in a different behaviour when compared with euglycemic subjects. Indeed, to the best of our knowledge, this is the first study to analyse the GLP-1/GLP-1R axis in AT of subjects with these characteristics. Our results lead us to speculate that our patient cohort might present disrupted GLP-1/GLP-1R signalling, resulting in a decrease in GLP-1 sensitivity in AT. This hypothesis might explain why those patients with greater BMI and levels of insulin, who would be expected to have the lowest circulating GLP-1 concentrations and a higher expression of the receptor, showed the opposite; that is, a lower receptor expression at the VAT level.
Regarding the relationship between GLP-1R expression in AT and GLP-1 concentration/secretion, we were unable to find an association before surgery. However, in subjects who underwent mRYGB, who showed the greatest incretin response after surgery, GLP-1R expression in AT negatively predicted the increase in GLP-1 concentration after the procedure. Although this finding has to be interpreted with caution due to the small sample size, it might suggest the existence of a regulatory feedback between GLP-1 secretion and GLP-1R expression in VAT. The absence of an association in the other bariatric procedures could be due to the lower improvement in the incretin response observed in non-derivative surgeries.
GLP-1R has been associated with both lipogenic and lipolytic activity5,6,7,8,9,10,11,12,13,14,15. In the present study, however, no association was found between GLP-1R expression and plasma lipid profile before or after surgery. Nevertheless, we only determined plasma circulating lipids and not intra-adipocyte lipolysis.
Many hormonal and clinical factors have been implicated in glucose improvement and weight loss after bariatric surgery37,38,39. To our knowledge, however, this is the first study analysing the predictive value of pre-surgery GLP-1R expression in AT on glucose metabolism and weight outcomes after different bariatric procedures. But, despite its potential relationship with incretin secretion, no association was found between GLP-1R expression in AT and either diabetes remission, insulin resistance improvement or weight loss after surgery.
We are aware that the study has some limitations. The limited number of subjects included and the low GLP-1R expression levels detected may reduce the strength of our observations. Moreover, the fact that AT biopsy samples were only available during surgery, and not afterwards, prevents the evaluation of GLP-1R expression changes with metabolic improvement after bariatric surgery, thus limiting our results. Also, we could only analyse glucagon concentrations in the fasting state, and so we could not calculate the AUC for this hormone, which is a more reliable indicator of its secretion. As our study was exploratory, further additional dedicated studies should be conducted in larger cohorts with pre-planned hypotheses and higher statistical power to confirm the observed associations.
Conclusions
In subjects with morbid obesity and T2D, GLP-1R expression in AT is of limited value to predict incretin response and does not play a role in metabolic outcomes such as insulin sensitivity improvement, T2D remission and weight loss after bariatric surgery. Further studies are warranted to explore a possible paracrine role of this receptor in AT.
Data Availability
All data analysed during this study is included in this article as well as in a previous published article cited in the references. If any further information is required please contact the corresponding author.
References
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol Rev 87, 1409–1439 (2007).
Nauck, M. A. et al. Glucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetes. Exp Clin Endocrinol Diabetes 105, 187–195 (1997).
Burcelin, R., Gourdy, P. & Dalle, S. GLP-1-based strategies: a physiological analysis of differential mode of action. Physiology (Bethesda) 29, 108–121 (2014).
Cantini, G., Mannucci, E. & Luconi, M. Perspectives in GLP-1 research: new targets, new receptors. Trends Endocrinol Metab 27, 427–438 (2016).
Majumdar, I. D. & Weber, H. C. Gastrointestinal regulatory peptides and their effects on fat tissue. Curr Opin Endocrinol Diabetes Obes 17, 51–56 (2010).
Ruiz-Grande, C. et al. Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides 13, 13–16 (1992).
Perea, A. et al. GLP-1 (7–36) amide: effects on glucose transport and metabolism in rat adipose tissue. Horm Metab Res 29, 417–421 (1997).
Vendrell, J. et al. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 152, 4072–4079 (2011).
Pastel, E. et al. Effects of Exendin-4 on human adipose tissue inflammation and ECM remodeling. Nutr Diabetes 6, e235 (2016).
Villanueva-Peñacarrillo, M. L. et al. Effect of GLP-1 on lipid metabolism in human adipocytes. Horm Metab Res 33, 73–77 (2001).
Azuma, K. et al. Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab 93, 459–464 (2008).
El Bekay, R. et al. Effects of glucagon-like peptide-1 on the differentiation and metabolism of human adipocytes. Br J Pharmacol 173, 1820–1834 (2016).
Challa, T. D. et al. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J BiolChem 287, 6421–6430 (2012).
Yang, J. et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int J Mol Med 31, 1429–1435 (2013).
Liu, R. et al. Glucagon like peptide-1 promotes adipocyte differentiation via the Wnt4 mediated sequestering of beta-catenin. PLoS One 9(11), e0160212 (2016).
Góralska, J. et al. Glucagon-like peptide-1 receptor agonist stimulates mitochondrial bioenergetics in human adipocytes. Acta Biochim Pol 64, 423–429 (2017).
Xu, F. et al. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 59, 1059–1069 (2016).
Hoffstedt, J. et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53, 2496–2503 (2010).
Björntorp, P. Metabolic difference between visceral fat and subcutaneous abdominal fat. Diabetes Metab 26(Suppl 3), 10–2 (2000).
Ibrahim, M. M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11, 11–18 (2010).
Wajchenberg, B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21, 697–738 (2000).
Buse, J. B. et al. How do we define cure of diabetes? Diabetes Care 32, 2133–2135 (2009).
Laferrère, B. et al. Incretin Levels and Effect Are Markedly Enhanced 1 Month After Roux-en-Y Gastric Bypass Surgery in Obese Patients With Type 2 Diabetes. Diabetes Care 30, 1709–1716 (2007).
Neville, M. J. et al. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity (Silver Spring) 19, 888–892 (2011).
Ceperuelo-Mallafré, V. et al. Disruption of GIP/GIPR axis in human adipose tissue is linked to obesity and insulin resistance. J Clin Endocrinol Metab 99, E908–19 (2014).
Casajoana, A. et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg 27, 2235–2245 (2017).
Wolever, T. M. et al. The glycemic index: methodology and clinical implications. Am J Clin Nutr 54, 846–854 (1991).
Gao, H. et al. GLP-1 amplifies insulin signaling by up-regulation of IR beta, IRS-1 and Glut4 in 3T3-L1 adipocytes. Endocrine 32, 90–95 (2007).
He, X. et al. Exendin-4 modifies adipogenesis of human adipose-derived stromal cells isolated from omentum through multiple mechanisms. Int J Obes (Lond) 42, 1051–1061 (2018).
Shen, W. R. et al. The Glucagon-Like Peptide-1 Receptor Agonist Exendin-4 Inhibits Lipopolysaccharide-Induced Osteoclast Formation and Bone Resorption via Inhibition of TNF-α Expression in Macrophages. J Immunol Res 13(2018), 5783639 (2018).
Lee, Y. S. et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55, 2456–68 (2012).
Krasner, N. M., Ido, Y., Ruderma, N. B. & Cacicedo, J. M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One 9, e97554 (2014).
Iacobellis, G. et al. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res 49, 625–630 (2017).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 18(108), 2460–2466 (2003).
Misra, A. & Vikram, N. K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 19, 457–466 (2003).
Lee, Y. S. & Jun, H. S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm 2016, 3094642 (2016).
Panunzi, S. et al. Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 39, 166–174 (2016).
Still, C. D. et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2, 38–45 (2014).
Batterham, R. L. & Cummings, D. E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 39, 893–901 (2016).
Acknowledgements
The authors thank Dr. Kenneth McCreath for helpful discussions on the manuscript and Bernat Miguel Huguet for statistical analysis. NV is the recipient of the grant “Ajuts per a projectes de recerca clínica de l′Hospital Universitari de Bellvitge (2011-PR143/11)” and of projects “PI11/01960, PI14/01997&PI17/01556” funded by the Instituto de Salud Carlos III (ISCIII) and co-funded by the European Regional Development Fund (ERDF, “A way to build Europe”). JV receives funding from ISCIII through the projects “PI14/00228 &PI17/01503” co-funded by the ERDF. SP has funding from ISCIII through projects “PI14/00633 & PI17/00915” co-funded by the ERDF. SFV has funding from the Spanish Ministry of Economy and Competitiveness “SAF2015-65019-R” and the ERDF. The Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders (CIBERDEM) (CB07708/0012) is an initiative of the ISCIII. SFV acknowledges support from the Miguel Servet tenure-track program (CP10/00438 and CPII16/0008) from the Fondo de Investigación Sanitaria, co-financed by the ERDF. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Author information
Authors and Affiliations
Contributions
Collected and analysed the data: M.E., F.G.P., N.M., E.M.-M., N.V. and R.L.U. Wrote the manuscript: M.E., F.G.P. and N.V. Gave suggestions on this study: J.P.G., A.G.G., M.P.M. and S.P. Conceived and designed the study: N.V., J.V., S.F.V. and A.C.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ejarque, M., Guerrero-Pérez, F., de la Morena, N. et al. Role of adipose tissue GLP-1R expression in metabolic improvement after bariatric surgery in patients with type 2 diabetes. Sci Rep 9, 6274 (2019). https://doi.org/10.1038/s41598-019-42770-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42770-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.