Medical Care
Actinic keratoses may remain unchanged, spontaneously resolve, or progress to invasive squamous cell carcinoma. [1] The fate of any one actinic keratosis is impossible to predict. Although the risk of progression of any one actinic keratosis to invasive squamous cell carcinoma is small (at most approximately 10%), [18] a patient may have many lesions, and thus the risk of progression becomes significant. Additionally, actinic keratoses can be clinically indistinguishable from more serious cutaneous malignancies, including squamous cell carcinoma and lentigo maligna. [39, 40] Therapy is generally well tolerated and simple; therefore, treatment of all actinic keratoses is warranted.
The appropriate treatment is generally chosen based on the number of lesions present and the efficacy of the treatment. [41] Additional variables to consider include persistence of the lesion(s), age of the patient, history of skin cancer, and tolerability of the treatment modality. [18] Treatment consists of 2 broad categories: surgical destruction of the lesion and medical therapy. Medical management begins with educating the patient to limit sun exposure. Patients should be cautioned to avoid sun exposure from 10:00 am to 3:00 pm as much as possible. They also must wear adequate sunscreens and protective clothing daily. [42]
Medical therapy has the advantage of being able to treat large areas with many lesions. The disadvantages of medical therapies include lengthy courses of treatment with irritation and discomfort. The US Food and Drug Administration (FDA) has approved six medications for the treatment of actinic keratoses. These are topical 5-fluorouracil (5-FU), 5% and 3.75% imiquimod cream, topical diclofenac gel, ingenol mebutate, tirbanibulin topical, and PDT with topical delta-aminolevulinic acid. [11, 12, 13, 43]
5-Fluorouracil
The most experience in topical therapy for actinic keratoses is with 5-FU, known to inhibit thymidylate synthetase and cause cell death in actively proliferating cells. [44] Several formulations are available, including a 5% cream or solution, a 2% solution, a 1% cream or solution, and, most recently, a micronized 0.5% cream. [11] Although not well studied, efficacy among the various formulations does not seem to differ significantly. [45, 46]
The most popular formulation is the 5% cream, which is applied twice daily for 1 month. During the treatment phase, the lesions become increasingly erythematous and cause discomfort; small subclinical lesions become visible. This treatment can be temporarily disfiguring, with erythematous ulcerations and crust formation. However, if the patient completes the treatment, the lesions usually heal within 2 weeks of stopping treatment, the complexion is smooth, and the actinic keratoses are improved.
The 0.5% micronized cream was developed to increase tolerability because inflammation and discomfort can be a limiting factor in the use of topical 5-FU. The 0.5% micronized cream is applied once daily for 1 month.
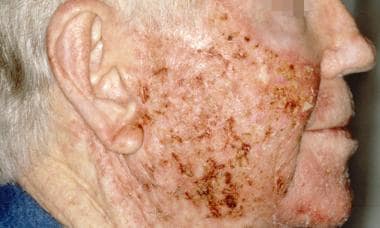
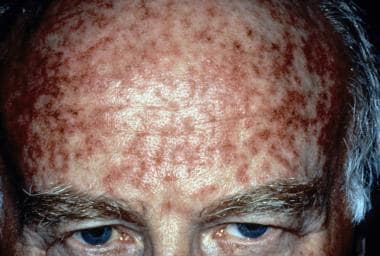
Usage of the 0.5% micronized cream 1 week prior to cryosurgery has also been shown to produce complete lesion clearance in a higher number of patients compared with cryosurgery alone (32.4% and 15%, respectively). [47] Note the images below.
Imiquimod
Imiquimod is a topical medication that up-regulates a variety of cytokines, which, in turn, invoke a nonspecific immune response (interferons, natural killer cells) and a specific immune response (T cells). It is applied 2-3 times a week for up to 4 months, although generally 1 month is sufficient. [48] Reaction to the drug is idiosyncratic, with some patients barely reacting and others developing marked inflammation. Subclinical lesions previously not appreciated may become inflamed during therapy. In patients with a brisk inflammatory response, dosing is reduced to twice or even once a week, with preservation of therapeutic efficacy but increased tolerability.
Two new formulations of imiquimod (2.5% and 3.75%) were studied and found to be efficacious in clearing actinic keratoses (25% and 34% clearance, respectively), with a more intuitive daily dosing schedule. Although not as efficacious as 5% imiquimod, the new formulations cause less irritation, promoting better compliance. [49] Experimental evidence suggests patients may develop T-cell memory after treatment with this drug and thus may be less likely to develop new actinic keratoses in the future. Imiquimod 5% cream has also been shown to be safe and effective in transplantation patients. [50, 51]
Ingenol mebutate topical
Ingenol mebutate gel (Picato) was approved by the FDA for actinic keratosis in January 2012 as a 2-3 day course of therapy. The dosage for actinic keratosis differs depending on the application site. The 0.015% gel is used for application to the face or scalp for 3 consecutive days, whereas the 0.05% gel is used for application to the trunk or extremities for 2 consecutive days.
Four multicenter, randomized, double-blind studies showed that ingenol mebutate gel applied topically for 2-3 days is effective for field treatment of actinic keratoses. The studies reported adverse effects that were generally mild to moderate in intensity and resolved without sequelae. [52] A phase III randomized, double-blind study displayed long-term benefit of ingenol mebutate 0.015% gel for both initial and follow-up therapy. [53] A posthoc analysis of patient-reported outcomes from phase III trials using ingenol mebutate gel for actinic keratosis found that treatment with this gel improved patients’ quality of life and treatment satisfaction, owing to higher degrees of lesion clearance. [54]
Although a precise mechanism of action has not been defined, a dual mechanism of action was described by Rosen et al. This dual mechanism includes (1) rapid lesion necrosis by mitochondrial swelling and membrane disruption and (2) specific neutrophil-mediated, antibody-dependent cellular cytotoxicity by antibodies produced from B cells that bind to antigens on dysplastic epidermal cells. [55]
Follow-up study supports these findings. [56, 57]
Jim On et al investigated the hypothesis that ingenol mebutate gel targets cell death in proliferating keratinocytes versus healthy skin by studying the local skin reaction (LSR) score after two cycles of treatment. The study included 20 participants with actinic keratoses on their face and scalp who applied 0.015% gel once daily for 3 days in two sequential 4-week cycles. The study found that the composite LSR score was significantly lower during the second cycle, thus suggesting that ingenol mebutate gel provides targeted therapy of actinic keratoses over cumulative dosing. [58]
In January 2020 as a precautionary measure, the European Medicines Agency (EMA) suspended the marketing authorization for ingenol mebutate (Picato) and recommended patients stop using the medication. [59] The EMA noted a trial that compared imiquimod and ingenol mebutate and found that skin cancer was more common in areas treated with ingenol mebutate versus imiquimod. The EMA notes that alternative treatments are available. The US Food and Drug Administration is investigating but has not made the recommendation to suspend use.
Topical diclofenac sodium 3% gel
Topical diclofenac sodium 3% gel is a nonsteroidal anti-inflammatory drug approved by the FDA for the treatment of actinic keratosis. Its mechanism of action against actinic keratoses is unknown. It is effective therapy when applied twice a day for 3 months. A shorter course of therapy is dramatically less effective. Its chief advantage is that it produces little-to-no inflammation and thus is very well tolerated. Diclofenac therapy after cryosurgery has also been shown to produce complete lesion clearance in a higher number of patients compared with cryosurgery alone (64% vs 32%, respectively). [60]
Tirbanibulin topical
Tirbanibulin topical is a novel microtubule inhibitor approved by the FDA in December 2020 for the treatment of actinic keratosis.
Approval was based on two phase III clinical trials (AK003 and AK004) that evaluated the safety and efficacy of tirbanibulin topical in adults (N=702) with facial or scalp actinic keratosis. The tirbanibulin-treated group achieved a higher percentage of complete clearance of actinic keratosis at Day 57 (AK003 [44% vs 5%] and AK004 [54% vs 13%]) and partial clearance compared with placebo in both trials (AK003 [68% vs 16%] and AK004 [76% vs 20%]). [43]
Photodynamic therapy
PDT uses a light-sensitizing compound that preferentially accumulates in actinic keratosis cells, where it can be activated by the appropriate wavelength of light. Delta-aminolevulinic acid is a component of the heme biosynthetic pathway that accumulates preferentially in dysplastic cells. Once inside these cells, it is enzymatically converted to protoporphyrin IX, a potent photosensitizer. With exposure to light of an appropriate wavelength, oxygen free radicals are generated and cell death results. [14]
Patients experience pain, similar in scope to the pain resulting from topical 5-FU, in the areas treated. The treated lesions may become erythematous and crusted. One treatment with PDT appears to be as effective as topical 5-FU therapy. [61]
A recent meta-analysis and systematic review assessed the effectiveness of PDT versus cryotherapy and found that PDT had a 14% better chance of complete lesion clearance at 3 months for thin actinic keratoses on the face and scalp compared with cryotherapy. [62]
Immunosuppressed patients may also benefit from PDT in the prevention of nonmelanoma skin cancers. [63]
When used with light sources that have a cosmetic benefit by themselves, such as the pulsed dye laser or intense pulsed light devices, a cosmetic benefit may be seen from the use of topical PDT beyond that of actinic keratosis eradication. Compared with other destructive therapeutic options such as cryotherapy, PDT may offer better cosmetic results and higher patient preference. [63, 64]
An unknown parameter in the use of topical PDT is the optimal incubation time following application of the topical aminolevulinic acid before light exposure. A second unknown parameter is the optimal light source to use for this treatment. Ongoing studies are addressing these issues. [63, 65]
Another photosensitizing agent approved in the use of PDT is methyl-5-aminolaevulinate (MAL). Comparison studies between ALA and MAL are not currently decisive. A study investigating the clinical efficacy of ALA- versus MAL-PDT in the treatment of actinic keratosis, Bowen disease, nodular basal cell carcinoma, and superficial basal cell carcinoma found that there were no statistically significant differences in their treatment outcomes using either of these agents. [66] However, a randomized, multicenter, observer-blind, placebo-controlled trial comparing the efficacy of BF-200 ALA versus MAL cream in the treatment of actinic keratoses lesions demonstrated that PDT with BF-200 ALA was superior to the MAL cream regarding patient complete clearance of lesions. Six- and 12-month follow-up studies substantiated the efficacy of PDT with BF-200 ALA and the lower recurrence rates of lesions with BF-200 ALA treatment versus MAL treatment. [67, 68] Looking beyond lesion clearance, MAL -PDT was found to be less painful when compared with ALA-PDT in a retrospective monocentric study of 173 patients. [69]
Surgical Care
The goal of surgical therapy is complete eradication of the actinic keratoses, usually by physical destruction, with limited-to-no damage to surrounding healthy tissue. When the diagnosis is unclear and an invasive tumor is possible, biopsy is indicated. However, biopsy generally leaves a scar.
Cryosurgery refers to use of a cryogen to lower the temperature of the skin and produce cell death. The most common cryogen used is liquid nitrogen, with a temperature of -195.8°C. Keratinocytes die when exposed to approximately -40 to -50°C. Other structures in the skin, such as collagen, blood vessels, and nerves, are more resistant to the lethal effects of cold than keratinocytes. Melanocytes are more sensitive than keratinocytes; thus, cryosurgery often leaves white spots. This technique has not been studied in a scientific fashion until 2004, when it was demonstrated to produce an overall clearance rate of 67-88%. [64, 70]
Lesions suggestive of invasive cancer may be treated with curettage, shave excision, or conventional excision, all of which provide a sample for histologic evaluation. These treatments require local anesthesia, produce a wound that requires time to heal, and are likely to scar.
Cosmetic resurfacing procedures, in which the entire epidermis is removed, sometimes with some portion of the dermis, are effective for actinic keratosis eradication. Cosmetic resurfacing procedures include medium and deep chemical peels, dermabrasion, and ablative laser resurfacing. [15, 16, 17] All of these are cosmetic procedures unlikely to be covered by insurance, all carry the risk of scarring and infection, and all require experience and expertise on the part of the dermatologic surgeon. They are highly unlikely to be performed solely for actinic keratosis therapy.
Diet
One study has suggested that a low-fat diet in humans leads to greater resolution of existent actinic keratoses and the development of fewer new ones during the study period. [33]
Activity
Instruct patients to practice sun safety, such as the use of sunscreen and protective clothing, and to limit outdoor activity from 10:00 am to 3:00 pm. [42]
Prevention
The development of these lesions is directly proportional to sun exposure. Actinic keratoses can be reduced or delayed by using sunscreens and reducing sun exposure. [42] Patients should limit recreational exposure, and those who work outdoors should consider making adjustments in their work-related sun exposure. For patients forced to undergo sun exposure, recommend applying a sunscreen of sun protection factor (SPF) 30 or more and wearing protective clothing daily. [42]
Long-Term Monitoring
If the lesions do not respond to topical therapy, they can be treated with cryotherapy with liquid nitrogen spray for 5-20 seconds. [70] Lesions become irritated and ulcerate, and eventually the diseased pathology is sloughed from healthy skin. [70]
A biopsy of more advanced lesions that are indurated should be performed to rule out an invasive carcinoma. [9]
-
Actinic keratosis. Courtesy of Hon Pak, MD.
-
Actinic keratosis during treatment with topical 5-fluorouracil. Courtesy of Hon Pak, MD.
-
Actinic keratosis right after treatment with topical 5-fluorouracil. Courtesy of Hon Pak, MD.
-
Erythematous, scaly lesions on the temple area, typical of actinic keratosis.