Reference Range
Immunofixation consists of an electrophoresis phase and a fixation phase. [1] Serum or urine immunofixation negative for a monoclonal protein or a polyclonal pattern is considered to be normal. Cerebral spinal fluid (CSF) immunofixation that does not reveal oligoclonal bands is also considered normal. A polyclonal immunoglobulin pattern in the serum or urine immunofixation is considered to be nonspecific.
Interpretation
Immunofixation can either reveal a normal pattern or identify a monoclonal protein or a polyclonal immunoglobulin pattern.
A normal result includes a darker immunoglobulin G (IgG) lane, a lighter immunoglobulin A (IgA), an absent immunoglobulin M (IgM), and a denser kappa compared to lambda lane, with ratio of 2:1. In a normal result, the lanes are broad and there is a gradual and smooth reduction in the color density toward the edges of the lane with no narrow dense band with sharp borders identified within the lane.
In some cases, all the lanes are homogeneously darkened to the same degree. This pattern represents the presence of polyclonal immunoglobulin. Again, the lanes are broad and the transition to the lane borders is smooth. In this case, the IgM lane, which is normally absent, is broad with smooth borders.
When a narrow band with sharp borders can be identified, it implies the presence of a monoclonal protein (see image below).
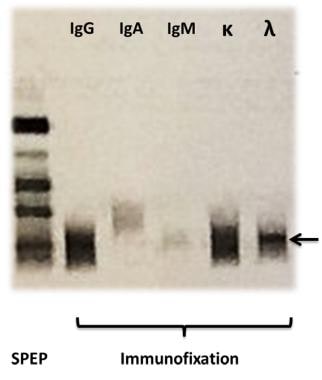 On the left a serum protein electrophoresis (SPEP) of a patient with a monoclonal band in the gamma region is shown. Subsequent immunofixation with electrophoresis identified the monoclonal protein as an IgG antibody (paraprotein) with a lambda light chain (arrow).
On the left a serum protein electrophoresis (SPEP) of a patient with a monoclonal band in the gamma region is shown. Subsequent immunofixation with electrophoresis identified the monoclonal protein as an IgG antibody (paraprotein) with a lambda light chain (arrow).
Finally, when several bands with sharp borders are seen after immunofixation in cerebrospinal fluid (CSF) but not in serum, the result is reported as positive for oligoclonal bands.
The identification of a monoclonal immunoglobulin is helpful in the diagnosis of the following conditions [2] :
-
Multiple myeloma [4, 5, 6]
-
Amyloidosis [8]
The identification of oligoclonal bands in CSF is helpful in the diagnosis of multiple sclerosis. [9]
In addition, immunofixation can be used to monitor therapy in plasma cell dyscrasias (ie, multiple myeloma and Waldenstrom macroglobulinemia). If the monoclonal protein level decreases or is undetectable after chemotherapy, it might indicate a response to treatment. On the contrary, a persistent monoclonal protein despite treatment is a sign of refractory disease. [10]
The presence of oligoclonal bands in CSF but not in the serum, given their intrathecal production, is helpful in the diagnosis of multiple sclerosis. Other inflammatory conditions of the CNS can present with oligoclonal bands in CSF resulting from intrathecal production of antibodies. [11] Human immunodeficiency virus (HIV)–related encephalitis, neurosyphilis, Lyme meningoencephalitis, neurosarcoidosis, acute disseminated encephalomyelitis (ADEM), systemic lupus erythematosus (SLE), Sjögren syndrome, subacute sclerosing panencephalitis, CNS malignancy, neuromyelitis optica, and transverse myelitis can all reveal oligoclonal banding in CSF immunofixation, precluding the use of immunofixation for the detection of oligoclonal bands as a sole diagnostic technique in multiple sclerosis.
Collection and Panels
The specimen can be serum, urine, or CSF.
Serum
Sample: Serum (whole blood or plasma is inappropriate, as fibrinogen may alter the results)
Tube: Red top (see image below)
Technique: Venipuncture
Sample size: 4 mL
Urine
Sample: 24-hour urine collection, concentrated
Collection in a container: A plastic collection container is used. The patient urinates each time into a clean dry vessel and then pours the urine in the plastic container, which is kept at a cold temperature (eg, in the refrigerator).
Technique: Collection starts early in the morning after the first morning urine has been voided and continues for the subsequent 24 hours, including the first morning urine of the following day.
Sample size: Every drop of urine within 24 hours is included in the sample.
Cerebrospinal fluid
Sample: CSF
Tube: CSF conical tube
Technique: Lumbar puncture
Sample size: 1 mL
Background
Description
Immunofixation consists of an electrophoresis phase and a fixation phase. [1] In the former, the serum is applied to an agarose gel, and the negatively charged serum proteins move toward the cathode under the influence of an electric current. The speed of movement is dictated by their charge. During the fixation phase, antiserum containing anti-IgG, anti-IgA, anti-IgM, anti-light chain kappa, or anti-light chain lambda is inoculated with the serum proteins. If a monoclonal protein is present, a precipitant will form at this phase. Finally, the sample is washed to remove the proteins that do not precipitate and then stained, destained, and dried.
In most cases in which a monoclonal protein is present, it shows as a narrow band at the IgG lane in combination with a monoclonal band at the kappa or lambda lane. IgA or IgM bands are less common. Finally, isolated light chain bands, either kappa or lambda, represent more commonly pure kappa or lambda light chain gammopathies or, less commonly, immunoglobulin D (IgD) or immunoglobulin E (IgE) gammopathies. Given the dismal impact on prognosis in the latter scenario, [12] an additional assay for the identification of IgD or IgE must be performed. If the result is negative, it is safe to conclude that a pure kappa or lambda light chain is present. [10]
Indications/Applications
Primary screening for plasma cell dyscrasias
No studies have addressed the role of serum or urine immunofixation in screening of the general population for multiple myeloma and related disorders. Therefore, such an approach cannot be currently recommended. [9]
Surveillance among patients with MGUS or smoldering (asymptomatic) multiple myeloma
Immunofixation of the urine and serum should be included in a battery of tests after 2-3 months following diagnosis of smoldering myeloma, then every 4-6 months for 1 year, and, finally, every 6-12 months if the results are stable. Surveillance for multiple myeloma in patients with MGUS and favorable prognostic factors (ie, low levels of monoclonal protein and IgG type) should include monitoring at 6 months and then every 2-3 years thereafter. For patients with MGUS and high risk for progression to multiple myeloma, surveillance should be performed at 6 months and then yearly thereafter. [13, 14]
Diagnosis of plasma cell dyscrasias [9]
Immunofixation is clearly indicated upon clinical or laboratory evidence of a plasma cell dyscrasia for the diagnosis of multiple myeloma, Waldenstrom macroglobulinemia, or amyloid light-chain (AL) amyloidosis. Typically, the monoclonal protein is discovered, and workup then ensues. These conditions should be promptly diagnosed and treated. In particular, when serum and urine protein electrophoresis results are negative, immunofixation is indicated as a more sensitive test if the clinical suspicion remains high. In addition, when protein electrophoresis assays are positive for a monoclonal protein, serum and urine immunofixation are indicated for the appropriate identification of the immunoglobulin and the corresponding light chain. However, most patients with a monoclonal protein will be finally diagnosed with MGUS rather than any of the malignant plasma cell dyscrasias for which treatment is recommended.
Follow-up of multiple myeloma, Waldenstrom macroglobulinemia, and AL amyloidosis
Immunofixation of urine and serum samples should be performed in all patients with multiple myeloma, Waldenstrom macroglobulinemia, and AL amyloidosis every 3 months after the completion of treatment in order to evaluate for response or to document a relapse. [15]
However, a study by Lahuerta et al indicated that, with the exception of persons with light-chain–only disease, urine immunofixation is not needed in combination with serum immunofixation to define complete response in multiple myeloma. Among 161 patients in whom both serum and urine demonstrated positive results for monoclonal protein and who were, posttreatment, negative for serum immunofixation, just 3 (1.9%) displayed positive urine immunofixation. [16]
Diagnosis of multiple sclerosis
Immunofixation in CSF for the detection of oligoclonal bands is indicated every time there is suspicion for multiple sclerosis. However, the presence of oligoclonal bands does not necessarily confirm the diagnosis, as other conditions can present with oligoclonal bands in the CSF.
Considerations
A negative urine or serum immunofixation result does not always rule out a plasma cell dyscrasia, as a nonsecretory or oligosecretory multiple myeloma can present with a negative immunofixation finding in both the urine and the serum. A serum free light chain ratio is indicated in the case of a negative immunofixation result when the suspicion for multiple myeloma is still high. [10, 17]
A 24-hour concentrated urine sample is appropriate for urine immunofixation. This sample is often difficult to collect, and suboptimal collection might alter the result. A concomitant measurement of the creatinine in the 24-hour urine sample for correction of sampling errors has been proposed. [18, 19]
Detection of a paraprotein by immunofixation is possible in lymphomas. [14]
Therapeutic monoclonal antibodies can interfere with results on SPEP and immunofixation. For example, a study by Scholl et al found that when casirivimab plus imdevimab, used in the treatment of coronavirus disease 2019 (COVID-19), was tested in serum samples, imdevimab produced “sustained assay interference (for at least 6 weeks) that could be misinterpreted as an IgG lambda monoclonal gammopathy.” [20]
Mass spectrometry
Mass spectrometry is sometimes employed in place of immunofixation because of the latter’s limitations, such as in the detection of low-level disease. Therefore, the International Myeloma Working Group Mass Spectrometry Committee created a set of recommendations for the evaluation of monoclonal proteins in multiple myeloma and related disorders, using mass spectrometry. Among these, the committee stated that in the detection of monoclonal immunoglobulins, intact light-chain matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry can be used in lieu of immunofixation in patient clinical assessments and in assessing clinical trial patients. [21]
Questions & Answers
Overview
What is the reference range of an immunofixation test?
How is an immunofixation test interpreted?
What is the clinical application of an immunofixation test?
What specimens can be used to perform an immunofixation test?
How is a serum sample collected for an immunofixation test?
How is a urine sample collected for an immunofixation test?
How is a CSF sample collected for an immunofixation test?
What is an immunofixation test?
When is an immunofixation test indicated?
When might mass spectrometry be used in place of immunofixation?
-
On the left a serum protein electrophoresis (SPEP) of a patient with a monoclonal band in the gamma region is shown. Subsequent immunofixation with electrophoresis identified the monoclonal protein as an IgG antibody (paraprotein) with a lambda light chain (arrow).
-
Red top tube for the collection of serum used. Image courtesy of Rush University.