Discussion
MRI is a superb diagnostic tool that is being used on an increasingly frequent basis. No long-term or irreversible biological effects have been associated with the radiation and magnetic fields, specifically, radiofrequency radiation, static magnetic, and magnetic pulsating gradient fields, that it generates.[13] However, there has been a concern that a large and growing number of cardiac PM recipients may suffer from the effects of these strong magnetic and radiofrequency fields. Thus, these patients have been systematically excluded from this diagnostic procedure based on theoretical concerns and demonstrated adverse interactions but with earlier generation of devices.
The effects of MRI on PM have been studied in vitro , as well as implanted in animal models and in humans.[3,4,911,13] Besides several complications, such as pacing inhibition, unpredictable programming changes, movement of the pulse generator inside the pocket, or permanent damage to the circuitry, the most potentially serious is inappropriately rapid pacing ("runaway" phenomenon) with induction of ventricular tachycardia or fibrillation. Radiofrequency pulses used during MRI have been associated with pacing at rates equal or multiple of their wavelength. In animals, Hayes and Vlietstra have observed pacing at >300 bpm, synchronized with the radiofrequency pulses, a phenomenon attributed to interference with the PM electronic circuitry.[13] Although the mechanism remains unclear, it is believed that the PM lead serves as an antenna, such that the radiofrequency energy pulses, along with the PM output circuit, stimulate the heart at rapid rates. [5,6]
The powerful static magnetic field inside the MRI scanner shield may close the reed switch and trigger pacing in asynchronous mode. However, the fluctuations in spatial orientation of the magnetic pulsating field gradient may also repeatedly open and close the reed switch and alternatively reset the PM in demand or asynchronous mode.[7] Programming of the pulse generator to AOO, VOO, or DOO modes has been recommended as the preferred prevention against sensing by the PM of interfering currents picked up by lead.[8]
Another potential complication of MRI scanning is heating of the PM and leads by current induction, due to radiofrequency energy transmission in the vicinity of the PM or through the leads, which act as antennas for the electromagnetic fields. Achenbach et al. reported that leads exposed to MRI in a 1.5 T system may reach temperatures up to 63.1°C during a 90 seconds exposure,[14] though the in vivo effects may be less prominent, since circulating blood serves as a heat sink to pull the heat away from the lead. In that study, placement of the leads in a saline bath when exposed to MRI fields markedly reduced the heating effect. Significant focal heating at the electrode-tissue interface has raised concerns about delayed effects, such as an increase in the pacing threshold, atrial or ventricular perforation, or reentrant arrhythmias due to endocardial scars. None of our patients reported discomfort that might be associated with PM or leads heating or movement inside the pocket, and there were no significant changes in the sensing and pacing thresholds, or impedance measurements observed after the MRI scan to suggest an endocardial lesion produced by heating of the endocardial electrodes. Although we did not rigorously exclude the presence of pericardial effusions, no patient reported signs and or symptoms consistent with such complication.
The current strength generated in the pacing lead during MRI scanning depends, to a great extent, on the lead's characteristics, including polarity and coaxial design versus parallel configuration, the intensity of the field applied, and the position of the lead relative to that field.[15] The Tendril model 1388 is a MP35N lead with coaxial configuration, which may predispose to electromagnetic interference, whereas its bipolar design will attenuate the relative contribution of the magnetic field. In our study, all PM were programmed in DDD mode, bipolar atrial and ventricular sensing, with the hysteresis function OFF, and the base and maximum ventricular tracking rates at 80 and 120 bpm, respectively. The base pacing rate was intentionally chosen so that it would be faster than the patient's sinus rate and slower than the magnet mode rate (100 bpm), facilitating an adverse interaction between the MRI study and the implanted system as manifested by significant pauses or reed switch activations. We observed neither pauses nor electrocardiographic abnormalities consistent with PM inhibition or asynchronous mode pacing. Further, there was no evidence of heart rate acceleration due to ventricular pacing in response to signals sensed in the atrial channel, nor high rate ventricular pacing produced by lead-generated currents.
It is possible that the patient's position in the MRI scanner, or our choice of specific exploration sequences, may have prevented the generation of currents strong enough to inhibit the PM or stimulate the heart. During scanning, all patients remained in the same position and underwent the usual and necessary sequence to complete the exploration as well as to determine the position of the implanted lead.
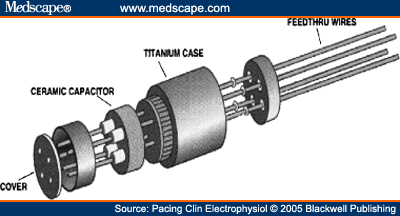
The PM manufactured by St. Jude Medical (Paragon®, Affinity®, Integrity®, Identity®, and FrontierTM) includes a ceramic condenser (Feed-through Ceramic Capacitor, Fig. 1) located in the header, which acts as a capacitive filter of signals at frequencies between 10 MHz and 10 GHz, and which dampens frequencies generated during the MRI scan. This condenser, together with the programming of bipolar sensing and inactivation of the magnet response, were the most likely mechanisms protecting these PM against electromagnetic interference during the scan.
Feed-through ceramic capacitor.
Pacing Clin Electrophysiol. 2005;25(2):274-278. © 2005 Blackwell Publishing
Cite this: Is Magnetic Resonance Imaging Safe in Cardiac Pacemaker Recipients? - Medscape - Apr 01, 2005.