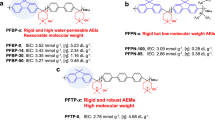
Abstract
One promising approach to reduce the cost of fuel cell systems is to develop hydroxide exchange membrane fuel cells (HEMFCs), which open up the possibility of platinum-group-metal-free catalysts and low-cost bipolar plates. However, scalable alkaline polyelectrolytes (hydroxide exchange membranes and hydroxide exchange ionomers), a key component of HEMFCs, with desired properties are currently unavailable, which presents a major barrier to the development of HEMFCs. Here we show hydroxide exchange membranes and hydroxide exchange ionomers based on poly(aryl piperidinium) (PAP) that simultaneously possess adequate ionic conductivity, chemical stability, mechanical robustness, gas separation and selective solubility. These properties originate from the combination of the piperidinium cation and the rigid ether-bond-free aryl backbone. A low-Pt membrane electrode assembly with a Ag-based cathode using PAP materials showed an excellent peak power density of 920 mW cm−2 and operated stably at a constant current density of 500 mA cm−2 for 300 h with H2/CO2-free air at 95 °C.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Yoshida, T. & Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Interface Magazine 24, 45–49 (2015).
Setzler, B. P., Zhuang, Z. B., Wittkopf, J. A. & Yan, Y. S. Activity targets for nanostructured platinum group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 11, 1020–1025 (2016).
Bashyam, R. & Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 443, 63–66 (2006).
Lefevre, M., Proietti, E., Jaouen, F. & Dodelet, J. P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Wu, G., More, K. L., Johnston, C. M. & Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 332, 443–447 (2011).
Gasteiger, H. A. & Markovic, N. M. Just a dream-or future reality? Science 324, 48–49 (2009).
Liang, Y. Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Varcoe, J. R. & Slade, R. C. T. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 5, 187–200 (2005).
Wang, Y. J., Qiao, J. L., Baker, R. & Zhang, J. J. Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 42, 5768–5787 (2013).
Varcoe, J. R. et al. Anion-exchange membranes in electrochemical energy systems. Energ. Environ. Sci. 7, 3135–3191 (2014).
Wang, J. H., Gu, S., Kaspar, R. B., Zhang, B. Z. & Yan, Y. S. Stabilizing the imidazolium cation in hydroxide-exchange membranes for fuel cells. ChemSusChem 6, 2079–2082 (2013).
Pan, J. et al. High-performance alkaline polymer electrolyte for fuel cell applications. Adv. Funct. Mater. 20, 312–319 (2010).
Gu, S. et al. A soluble and highly conductive ionomer for high-performance hydroxide exchange membrane fuel cells. Angew. Chem. Int. Ed. 48, 6499–6502 (2009).
Wang, J. H., Li, S. H. & Zhang, S. B. Novel hydroxide-conducting polyelectrolyte composed of an poly(arylene ether sulfone) containing pendant quaternary guanidinium groups for alkaline fuel cell applications. Macromolecules 43, 3890–3896 (2010).
Noonan, K. J. T. et al. Phosphonium-functionalized polyethylene: A new class of base-stable alkaline anion exchange membranes. J. Am. Chem. Soc. 134, 18161–18164 (2012).
Zha, Y. P., Disabb-Miller, M. L., Johnson, Z. D., Hickner, M. A. & Tew, G. N. Metal-cation-based anion exchange membranes. J. Am. Chem. Soc. 134, 4493–4496 (2012).
Gu, S. et al. Permethyl cobaltocenium (Cp*2Co+) as an ultra-stable cation for polymer hydroxide-exchange membranes. Sci. Rep. 5, 11668 (2015).
Hugar, K. M., Kostalik, H. A. & Coates, G. W. Imidazolium cations with exceptional alkaline stability: A systematic study of structure–stability relationships. J. Am. Chem. Soc. 137, 8730–8737 (2015).
Marino, M. G. & Kreuer, K. D. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem 8, 513–523 (2015).
Olsson, J. S., Pham, T. H. & Jannasch, P. Poly(arylene piperidinium) hydroxide ion exchange membranes: Synthesis, alkaline stability, and conductivity. Adv. Funct. Mater. 28, 1702758 (2017).
Peng, H. G. et al. Alkaline polymer electrolyte fuel cells stably working at 80 °C. J. Power Sources 390, 165–167 (2018).
Dang, H. S. & Jannasch, P. Alkali-stable and highly anion conducting poly(phenylene oxide)s carrying quaternary piperidinium cations. J. Mater. Chem. A 4, 11924–11938 (2016).
Pham, T. H. & Jannasch, P. Aromatic polymers incorporating bis-N-spirocyclic quaternary ammonium moieties for anion-exchange membranes. ACS Macro Lett. 4, 1370–1375 (2015).
Pham, T. H., Olsson, J. S. & Jannasch, P. N-spirocyclic quaternary ammonium ionenes for anion-exchange membranes. J. Am. Chem. Soc. 139, 2888–2891 (2017).
Strasser, D. J., Graziano, B. J. & Knauss, D. M. Base stable poly(diallylpiperidinium hydroxide) multiblock copolymers for anion exchange membranes. J. Mater. Chem. A 5, 9627–9640 (2017).
Olsson, J. S., Pham, T. H. & Jannasch, P. Poly(N,N-diallylazacycloalkane)s for anion-exchange membranes functionalized with N-spirocyclic quaternary ammonium cations. Macromolecules 50, 2784–2793 (2017).
Ponce-Gonzalez, J. et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 9, 3724–3735 (2016).
Arges, C. G. & Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl Acad. Sci. USA 110, 2490–2495 (2013).
Mohanty, A. D., Tignor, S. E., Krause, J. A., Choe, Y. K. & Bae, C. Systematic alkaline stability study of polymer backbones for anion exchange membrane applications. Macromolecules 49, 3361–3372 (2016).
Hibbs, M. R., Fujimoto, C. H. & Cornelius, C. J. Synthesis and characterization of poly(phenylene)-based anion exchange membranes for alkaline fuel cells. Macromolecules 42, 8316–8321 (2009).
Fujimoto, C., Kim, D. S., Hibbs, M., Wrobleski, D. & Kim, Y. S. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells. J. Membr. Sci. 423, 438–449 (2012).
Fujimoto, C. H., Hickner, M. A., Cornelius, C. J. & Loy, D. A. Ionomeric poly(phenylene) prepared by Diels–Alder polymerization: Synthesis and physical properties of a novel polyelectrolyte. Macromolecules 38, 5010–5016 (2005).
Lee, W. H., Mohanty, A. D. & Bae, C. Fluorene-based hydroxide ion conducting polymers for chemically stable anion exchange membrane fuel cells. ACS Macro Lett. 4, 453–457 (2015).
Lee, W. H., Kim, Y. S. & Bae, C. Robust hydroxide ion conducting poly(biphenyl alkylene)s for alkaline fuel cell membranes. ACS Macro Lett. 4, 814–818 (2015).
Pan, J. et al. Constructing ionic highway in alkaline polymer electrolytes. Energy Environ. Sci. 7, 354–360 (2014).
Tanaka, M. et al. Anion conductive block poly(arylene ether)s: Synthesis, properties, and application in alkaline fuel cells. J. Am. Chem. Soc. 133, 10646–10654 (2011).
Li, N. W., Yan, T. Z., Li, Z., Thurn-Albrecht, T. & Binder, W. H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 5, 7888–7892 (2012).
Li, N. W., Leng, Y. J., Hickner, M. A. & Wang, C. Y. Highly stable, anion conductive, comb-shaped copolymers for alkaline fuel cells. J. Am. Chem. Soc. 135, 10124–10133 (2013).
Wang, J. H. et al. Structure–property relationships in hydroxide-exchange membranes with cation strings and high ion-exchange capacity. ChemSusChem 8, 4229–4234 (2015).
Li, Y. F. et al. Polyethylene-based block copolymers for anion exchange membranes. Macromolecules 48, 6523–6533 (2015).
Cruz, A. R. et al. Precision synthesis of narrow polydispersity, ultrahigh molecular weight linear aromatic polymers by A2 + B2 nonstoichiometric step-selective polymerization. Macromolecules 45, 6774–6780 (2012).
Diaz, A. M. et al. A novel, one-pot synthesis of novel 3F, 5F, and 8F aromatic polymers. Macromol. Rapid Comm. 28, 183–187 (2007).
Pena, E. R., Zolotukhin, M. & Fomine, S. Factors enhancing the reactivity of carbonyl compounds for polycondensations with aromatic hydrocarbons. A computational study. Macromolecules 37, 6227–6235 (2004).
Cruz, A. R. et al. Use of 4-piperidones in one-pot syntheses of novel, high-molecular-weight linear and virtually 100%-hyperbranched polymers. Chem. Commun. 0, 4408–4410 (2009).
Wang, J. H., Zhao, Z., Gong, F. X., Li, S. H. & Zhang, S. B. Synthesis of soluble poly(arylene ether sulfone) ionomers with pendant quaternary ammonium groups for anion exchange membranes. Macromolecules 42, 8711–8717 (2009).
Kaspar, R. B. et al. Manipulating water in high-performance hydroxide exchange membrane fuel cells through asymmetric humidification and wetproofing. J. Electrochem. Soc. 162, F483–F488 (2015).
Dekel, D. R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 375, 158–169 (2018).
Wang, L. Q., Bellini, M., Miller, H. A. & Varcoe, J. R. A high conductivity ultrathin anion-exchange membrane with 500+ h alkali stability for use in alkaline membrane fuel cells that can achieve 2 W cm−2 at 80 °C. J. Mater. Chem. A 6, 15404–15412 (2018).
Peng, X. et al. Nitrogen-doped carbon–CoOx nanohybrids: A precious metal free cathode that exceeds 1.0 W cm−2 peak power and 100 h life in anion-exchange membrane fuel cells. Angew. Chem. Int. Ed. 2019, 1046 (2018).
Dekel, D. R. et al. Effect of water on the stability of quaternary ammonium groups for anion exchange membrane fuel cell applications. Chem. Mater. 29, 4425–4431 (2017).
Acknowledgements
This material is based upon work supported by the by the US Department of Energy, Advanced Research Projects Agency-Energy under Award No. DE-AR0000771. We thank Z. Green and H. Xu at Giner for performing the gas crossover measurement. We also thank R. Ma, J. Nash, J. A. Wittkopf and M. D. Woodroof for their assistance in the durability test of H2/O2 HEMFCs.
Author information
Authors and Affiliations
Contributions
J.W. synthesized the polymers, fabricated the membranes, and performed the characterizations of polymers and membranes. Y.Z. prepared MEAs with Pt electrodes and tested their H2/O2 performance and durability. B.P.S. measured the electronic area specific resistance and stability of the membrane under RH cycling and pressure differential. S.R.-C. and L.S. developed the casting process for fabricating the large membranes. C.B.Y., A.A. and M.P. prepared MEAs with Ag cathodes and low-Pt anodes and tested their H2/CO2-free air performance and durability. L.W. performed the thermal analysis for the membranes. K.H. measured the polymer molecular weight. S.G. provided guidance in the development and testing of H2/CO2-free air MEAs. J.W., Y.Z., B.X. and Y.Y. conceived the ideas and wrote the manuscript with support from other coauthors.
Corresponding authors
Ethics declarations
Competing interests
C.B.Y, A.A. and M.P. are employed by Elbit Systems Ltd, which is a company specializing in fuel cells.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13, Supplementary Tables 1–2
Rights and permissions
About this article
Cite this article
Wang, J., Zhao, Y., Setzler, B.P. et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat Energy 4, 392–398 (2019). https://doi.org/10.1038/s41560-019-0372-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0372-8
This article is cited by
-
Tuning the apparent hydrogen binding energy to achieve high-performance Ni-based hydrogen oxidation reaction catalyst
Nature Communications (2024)
-
Unveiling the nature of Pt-induced anti-deactivation of Ru for alkaline hydrogen oxidation reaction
Nature Communications (2024)
-
Implanting oxophilic metal in PtRu nanowires for hydrogen oxidation catalysis
Nature Communications (2024)
-
A fast ceramic mixed OH−/H+ ionic conductor for low temperature fuel cells
Nature Communications (2024)
-
An operationally broadened alkaline water electrolyser enabled by highly stable poly(oxindole biphenylene) ion-solvating membranes
Nature Energy (2024)