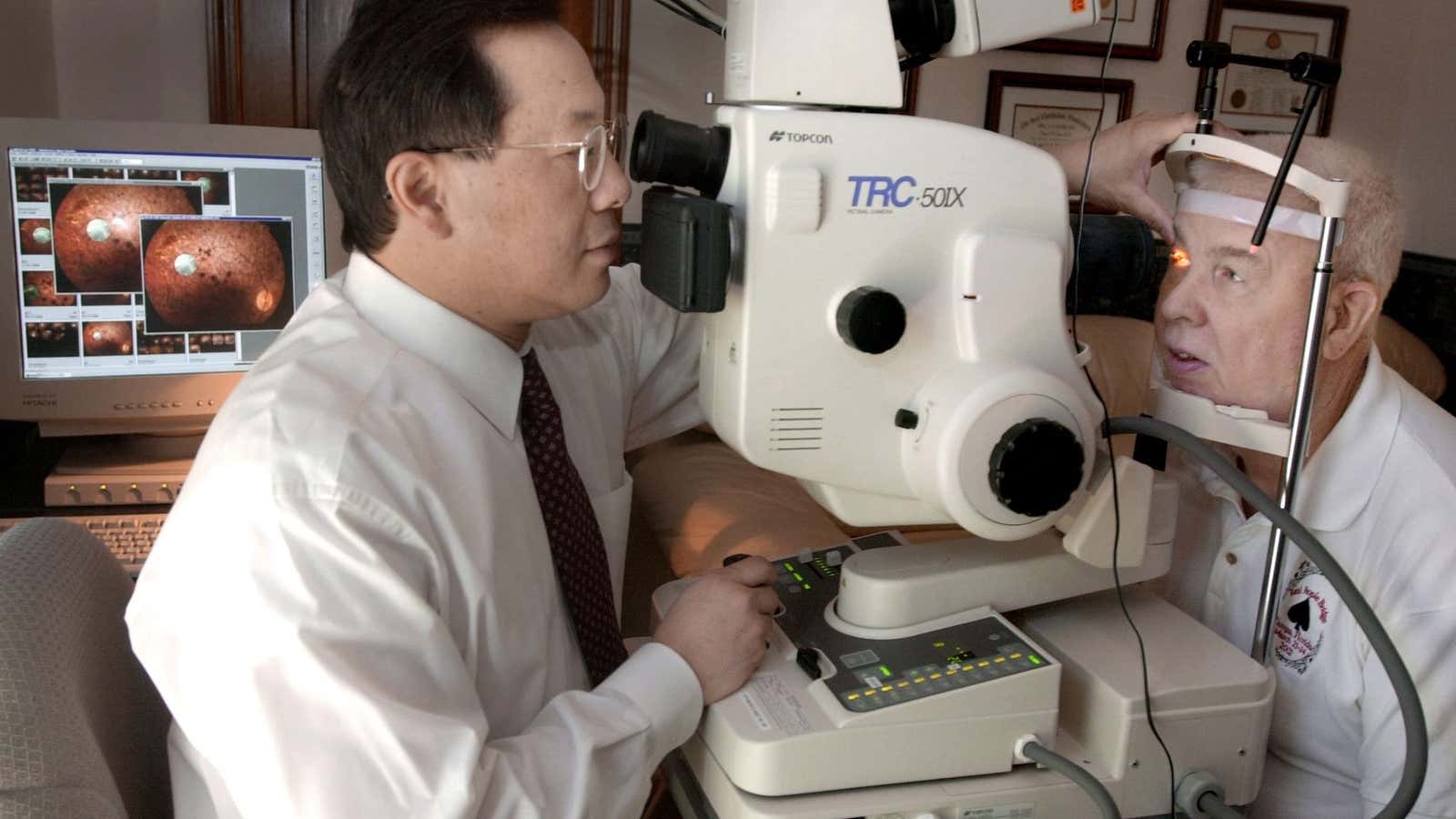
The US Food and Drug Administration approved this week the first software powered by artificial intelligence that replaces the need for a specialized doctor to interpret medical imagery.
The software is called IDx-DR, made by diagnostic AI startup IDx, and specifically analyzes images of the retina to detect whether a person with diabetes has a complication from the disease called diabetic retinopathy.
“IDx-DR is the first device authorized for marketing that provides a screening decision without the need for a clinician to also interpret the image or results, which makes it usable by health care providers who may not normally be involved in eye care,” the FDA wrote in a press release.
Diabetic retinopathy is a complication of diabetes where blood sugar damages the back of the eye, according to the FDA, and is the main cause of the loss of vision for those with diabetes.
To use the software, a doctor takes a retinal image with a specific model of camera and uploads it to IDx-DR’s cloud service. Once the image is determined to be high enough quality, the program tells the doctor that either significant retinopathy was detected, or that it didn’t detect retinopathy and to run the test again in a year.
By allowing this software to be marketed in the US, the FDA is setting a bar for the accuracy needed in order for AI to take over for human doctors. When validating that the AI system worked, the FDA used images from 900 US patients. The software correctly detected more than mild diabetic retinopathy 87.4% of the time, and identified when patients did not have more than mild retinopathy 89.5% of the time. Accuracy for humans naturally varies from doctor to doctor, but for the FDA to approve the technology it “must provide for more effective treatment or diagnosis of a life-threatening or irreversibly debilitating disease or condition.”
This is welcome news for large technology companies like IBM, Google, and Microsoft, which have all made large investments in the medical AI space. Google is expected to pay $100 million to collect data on 100,000 research participants, and IBM has spent more than $4 billion over the last few years gobbling up medical companies for their data and talent. The FDA approval is especially fortuitous for Google, which has used AI to extrapolate even more from retinal images—like whether someone has heart disease.