December 19, 2016
As the two finalist products in Qualcomm's Tricorder XPRIZE competition advance to consumer testing, the teams that developed them shed light on what it takes to bring Star Trek technology to the real world.
Kristopher Sturgis
|
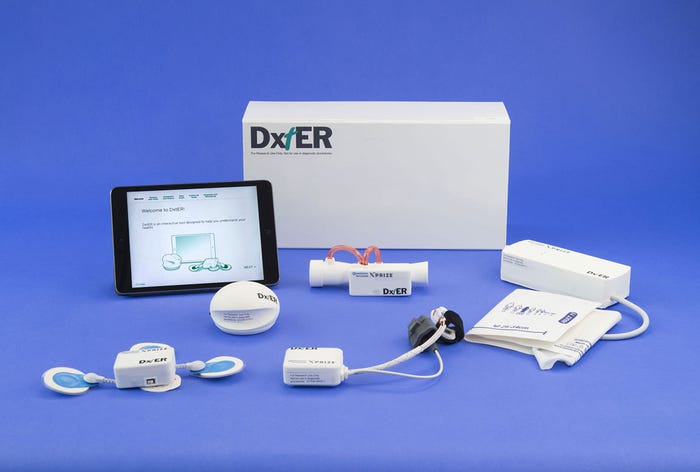
The DxtER prototype was one of two medical Tricorder concepts to advance to the final round of the Qualcomm Tricorder XPRIZE competition. |
Fifty years ago Star Trek introduced its viewers to a futuristic world with countless gadgets and technologies that captivated millions. Among their many creations was a handheld diagnostic scanning device known as the medical Tricorder, a multifunctional device that could be used to diagnose injury and disease through advanced sensor scanning technologies. Last week, XPRIZE announced the two devices based on the famous Star Trek technology that will move into live consumer testing as part of the final phase of the $10 million Qualcomm Tricorder XPRIZE competition.
The two devices weigh less than five pounds each, and were designed to measure five vital signs and test for 10 required core conditions as well as for the absence of other conditions. The two teams that have advanced to the final round of consumer testing are Dynamical Biomarkers Group, led by Harvard medical school professor Chung-Kang Peng, and Final Frontier Medical Devices, led by emergency medical physician Basil Harris. Both Harris and Peng said the biggest challenge when developing these devices was creating a device within the parameters of the competition itself.
"Probably the biggest challenge in developing this technology was the restrictive criteria set by the XPRIZE competition," Peng said. "The whole Tricorder set could not weigh more than five pounds, and yet the unit had to be reliable and user-friendly. In fact, we had to eliminate some heavier and more sophisticated components during the process of the competition as a result. However, the limitations were ironically liberating as well. We had to think outside the box, and having to operate within the confines of the competition rules forced us to think in more unconventional ways to establish a diagnosis."
"The timeline of the Qualcomm Tricorder XPRIZE is by design a huge challenge in itself," Harris said. "In part, it is how they accelerate the development of technology behind each XPRIZE. While science fiction provides the 'future vision', we developed the technology based on the needs of the devices we specified. The devices we built reflect the needs of the Diagnostic Engine, and the inputs it needs to distinguish between the diseases and conditions we want to detect. We tried to model off the vision of a non-invasive device that is able to deliver robust and reliable data."Harris agreed, adding that the timeline of the competition presented a challenge that actually accelerated the process of innovation, providing a new sense of drive.
|
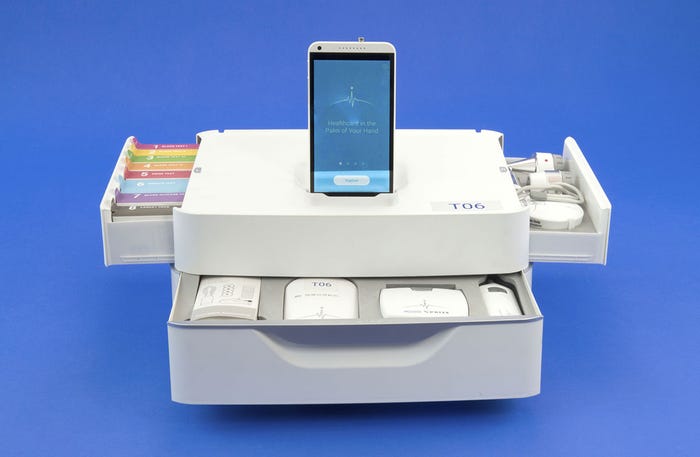
The Tricorder prototype from Dynamical Biomarkers Group features a scope set, a component for blood, urine, and breath tests, and a set for monitoring vital signs. |
Harris and his colleagues built a Tricorder prototype known as DxtER, a medical kit designed to be used by a person in their home, and can be operated without any medical or technical background. The device was built to operate completely autonomously without any external help or external interpretation of data by a medical professional. At the heart of the device lies a diagnostic engine that is based on analysis of actual patient data, which is what Harris believes sets their prototype apart.
"We have been working on this for the past several years and our Tricorder continues to evolve and gain capabilities," he says. "The challenges that remain include miniaturization, testing, and regulatory challenges. We are taking the prototype design through a miniaturization process so that the sensors can be incorporated into a watch band or clothing. The XPRIZE is a wonderful demonstration project for these prototypes, however rigorous testing and full scale clinical trials will be required in order to get these kinds of devices on the market."
As for Peng's group, their Tricorder unit was designed to incorporate three main components: a scope set; a component for blood, urine, and breath tests; and a set for monitoring vital signs. The scope set was designed to use a Bluetooth enabled magnifying camera that can capture high-resolution images of the skin. The blood, urine, and breath tests will allow the device to scan for a variety of different conditions from infections to diabetes, and the vital signs monitoring set will track everything from body temperature and blood pressure, to heart and respiratory rates--all packaged in a user-friendly device with state-of-the-art algorithms that can analyze physiological signals.
"This process took approximately three years from concept to generation," Peng said. "Prior to this competition, we had been doing preliminary work in the portable diagnosis of conditions such as atrial fibrillation and sleep apnea, but the idea of assembling a device capable of diagnosing up to 15 medical conditions--and diverse conditions at that--did not get initiated until the Qualcomm Tricorder Competition announcement in 2012. This competition pushed us to think broadly and ambitiously, and is a real tribute to what XPRIZE and Qualcomm are doing to push the boundaries of science."
Both Harris and Peng say that while their devices take on the final stage of consumer testing, both groups will continue to enhance their prototypes in an effort to broaden its impact and expand its capabilities. They both agree that before a device like this ever makes it to market, it needs to prove that it can be valuable and easy to use in the hands of a patient. Peng says that their device could have the biggest impact in rural and remote areas where access to medical care is limited. Harris agrees that Tricorder technologies in general could be especially useful in areas where there aren't enough medical professionals to support the population.
"We believe that Tricorder technology, or automated/technology-assisted diagnosis is in its infancy, and as it matures it will provide basic medical triage and basic medical diagnosis in support of medical personnel," Harris said. "Especially in areas where the number of medical professional can't support the population. This is true in many developing areas around the world, as well as in highly developed areas where access to healthcare remains a huge barrier. This is only the beginning of the evolution of this technology."
Both teams' devices are scheduled to undergo consumer testing over the next few months at the Altman Clinical Translational Research Institute at the University of California San Diego, with the winner being announced in the spring of 2017.
Kristopher Sturgis is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
[images courtesy of XPRIZE]
About the Author(s)
You May Also Like