Scientists find a way to create long-life, fast-charging batteries
A group of researchers led by Skoltech Professor Pavel Troshin studied coordination polymers, a class of compounds with scarcely explored applications in metal-ion batteries, and demonstrated their possible future use in energy storage devices with a high charging/discharging rate and stability. The results of their study were published in the journal Chemistry of Materials.
The charging/discharging rate is one of the key characteristics of lithium-ion batteries. Most modern commercial batteries need at least an hour to get fully charged, which certainly limits the scope of their application, in particular, for electric vehicles. The trouble with active materials, such as the most popular anode material, graphite, is that their capacity decays significantly, as their charging rate increases. To retain the battery capacity at high charging rates, the active electrode materials must have high electronic and ionic conductivity, which is the case with the newly-discovered coordination polymers that are derived from aromatic amines and salts of transition metals, such as nickel or copper. Although these compounds hold a great promise, their application in lithium-ion batteries remains virtually unexplored.
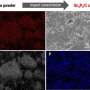
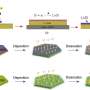
A recent study undertaken by a group of scientists from Skoltech and the Institute for Problems of Chemical Physics of RAS led by Professor P. Troshin in collaboration with the University of Cologne (Germany) and the Ural Federal University, focused on tetraaminobenzene-based linear polymers of nickel and copper. Although the linear polymers exhibited much lower initial electronic conductivity as compared to their two-dimensional counterparts, it transpired that they can be used as anode materials that get charged/discharged in less than a minute, because their conductivity increases dramatically after the first discharge due to lithium doping.
Additionally, it was found that these anode materials have excellent stability at high charging/discharging rates: they were demonstrated to retain up to 79% of their maximum capacity after as many as 20,000 charging-discharging cycles.
Furthermore, it was discovered that copper-based polymers can be used both as anode and high-capacity cathode materials. The authors point out that there is plenty of opportunity for structure optimization, even though the cathode cannot yet operate in a stable manner. "There are a lot of methods for fine-tuning the characteristics of coordination polymers," explains the first author of the study and Skoltech Ph.D. student, Roman Kapaev. "Actually we deal here with a sort of a construction kit where the parts can be easily changed or replaced. We can modify both the amine structure and the transition metal cation, and by doing so, raise the capacity, increase or decrease the redox potential, improve stability and various other performances. This trail-blazing study touches upon an extensive research area, which, I am sure, has yet a lot to reveal."
More information: Roman R. Kapaev et al, Nickel(II) and Copper(II) Coordination Polymers Derived from 1,2,4,5-Tetraaminobenzene for Lithium-Ion Batteries, Chemistry of Materials (2019). DOI: 10.1021/acs.chemmater.9b01366
Journal information: Chemistry of Materials
Provided by Skolkovo Institute of Science and Technology