At 70 least potential novel coronavirus vaccines are being developed by research teams across the globe, including in the U.S., U.K. and China, according to the latest report this month from the World Health Organization (WHO).
Several pharmaceutical companies and biotechnology firms have joined the race to find a vaccine for the COVID-19 virus, which has infected over 2.4 million people across at least 185 countries and regions, as of Tuesday, according to the latest figures from Johns Hopkins University.
The director of the U.S. National Institute of Allergy and Infectious Diseases (NIAID), Dr. Anthony Fauci, who is also a member of the White House COVID-19 task force, stated: "It will take at least a year to a year in a half to have a vaccine we can use."
Here we look at some of the main potential COVID-19 vaccine developments currently in the pipeline.
U.S.
Rearchers at Inovio Pharmaceuticals have developed the INO-4800 vaccine, which is given as a skin-deep shot instead of the typical deeper shot. The vaccine is in phase one of its clinical trials in Kansas City, Missouri, being conducted among 40 participants. The company is also working with China to begin studies there.
Earlier this month, Novavax (a Maryland-based biotechnology firm) announced its NVX-CoV2373 vaccine will begin its first human trial on 130 participants in mid-May, with preliminary results expected in July.
Pre-clinical studies found the vaccine "produces high levels of neutralizing antibodies against SARS-CoV-2 in animal studies," said Gregory Glenn, the president of research and development at Novavax.
"This is strong evidence that the vaccine created by Novavax has the potential to be highly immunogenic in humans which could lead to protection from COVID-19 and helping to control the spread of this disease," said Matthew Frieman, associate professor at the University of Maryland School of Medicine.
Johnson & Johnson, in collaboration with Beth Israel Deaconess Medical Center (part of Harvard Medical School), has been looking at potential vaccine candidates since January this year.
Last month, the researchers identified a lead vaccine candidate, which could potentially see a vaccine be available for emergency use by early 2021. Clinical trials are expected to begin in September, with data on the safety and efficacy of the vaccine expected to be available by the end of the year, the company confirmed in a statement.
"Our goal is to enable the supply of more than 1 billion doses of the vaccine globally," vice chairman of the executive committee and chief scientific officer at Johnson & Johnson, Paul Stoffels, told Yahoo earlier this month.
Johnson & Johnson previously developed the Ebola vaccine, while its vaccine candidates for Zika, RSV, and HIV are currently in the second and third phases of their clinical development stages.
Last month, the U.S. began the first human trial for a potential COVID-19 vaccine. Developed by the biotechnology firm Moderna, the first phase of the trial for the mRNA-1273 vaccine is being conducted at Seattle-based Kaiser Permanente Washington Health Research Institute (KPWHRI).
Emory University's VTEU (Vaccine and Treatment Evaluation Unit) in Atlanta, Georgia was added by the NIAID, which is funding the trial, as a second test site for the first phase of the study.
The trial will observe 45 participants over 14 months to test how various safe doses of the vaccine react and whether it kicks the immune system into action. The vaccine uses a segment of the virus' genetic code rather than a piece of the virus, which it is hoped will allow it to be developed more quickly.
"Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority. This Phase 1 study, launched in record speed, is an important first step toward achieving that goal," Fauci said in a NIAID statement last month.
U.K.
Earlier this month, Sarah Gilbert, a professor of vaccinology at Oxford University, claimed that a vaccine for the novel coronavirus could potentially be ready by this September.
Gilbert and researchers at Oxford University's Jenner Institute and Oxford Vaccine Group have been developing the ChAdOx1 nCoV-19 vaccine since January.
Working with a base vaccine for other similar coronaviruses, Gilbert's team managed to speed up a development process that would normally take around five years to around four months.
Earlier this week, she confirmed that her team is waiting for final safety tests and approvals for the clinical trials, which she hopes could begin by the end of this week. But she urged millions of doses of vaccine would have to be manufactured even before these trials are concluded.
"What we need from the [U.K.] government is support to help us accelerate the manufacturing," she said speaking on BBC One's The Andrew Marr Show.
"There aren't any manufacturing facilities in this country that at the moment can make very large amounts of the vaccine," she added.
Gilbert previously stated that she gives the vaccine an 80 percent chance of being successful based on evidence that she has seen.
China
China is looking at three potential vaccines, including one by Chinese biopharmaceutical company CanSino Biologics, developed in collaboration with the Beijing Institute of Biotechnology. The first phase of clinical trials for the team's Ad5-nCoV vaccine was launched last month.
Recruitment of volunteers for the second phase of clinical trials for the Ad5-nCoV vaccine has begun. "It's the world's first novel coronavirus vaccine to initiate Phase II clinical studies," Wu Yuanbin, an official from China's Ministry of Science and Technology, said at a press briefing earlier this month.
Another potential vaccine is being developed by Beijing-based Sinovac Research and Development Co., Ltd, while another is being studied by the Wuhan Institute of Biological Products and the Wuhan Institute of Virology. Both vaccine candidates were approved for the first phase of their clinical trials earlier this month.
The graphic below, provided by Statista, illustrates countries with the most confirmed COVID-19 cases.
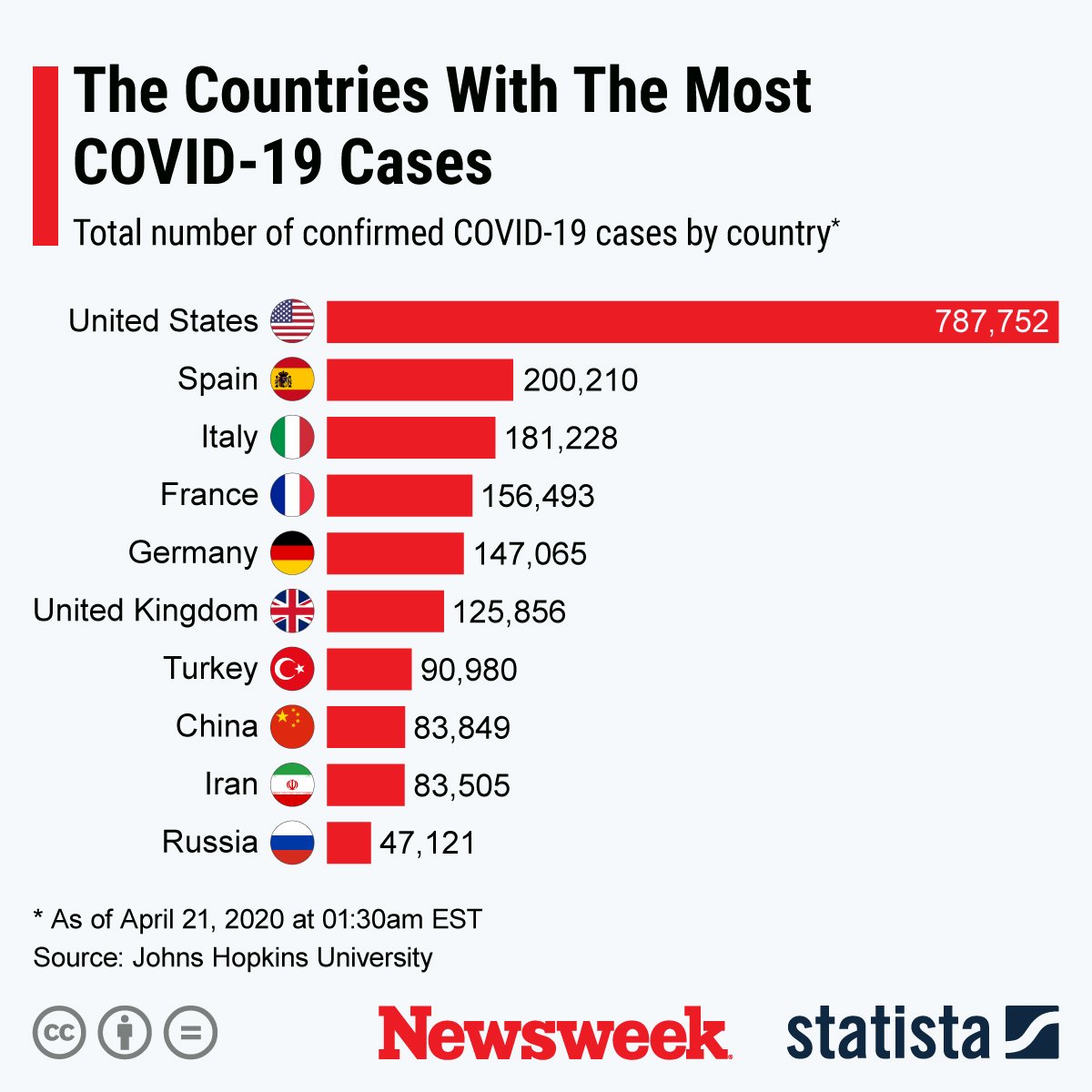
Data on COVID-19 cases is from Johns Hopkins University unless otherwise stated.
Centers for Disease Control and Prevention Advice on Using Face Coverings to Slow Spread of COVID-19
- CDC recommends wearing a cloth face covering in public where social distancing measures are difficult to maintain.
- A simple cloth face covering can help slow the spread of the virus by those infected and by those who do not exhibit symptoms.
- Cloth face coverings can be fashioned from household items. Guides are offered by the CDC. (https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html)
- Cloth face coverings should be washed regularly. A washing machine will suffice.
- Practice safe removal of face coverings by not touching eyes, nose, and mouth, and wash hands immediately after removing the covering.
World Health Organization advice for avoiding spread of coronavirus disease (COVID-19)
Hygiene advice
- Clean hands frequently with soap and water, or alcohol-based hand rub.
- Wash hands after coughing or sneezing; when caring for the sick; before, during and after food preparation; before eating; after using the toilet; when hands are visibly dirty; and after handling animals or waste.
- Maintain at least 1 meter (3 feet) distance from anyone who is coughing or sneezing.
- Avoid touching your hands, nose and mouth. Do not spit in public.
- Cover your mouth and nose with a tissue or bent elbow when coughing or sneezing. Discard the tissue immediately and clean your hands.
Medical advice
- Avoid close contact with others if you have any symptoms.
- Stay at home if you feel unwell, even with mild symptoms such as headache and runny nose, to avoid potential spread of the disease to medical facilities and other people.
- If you develop serious symptoms (fever, cough, difficulty breathing) seek medical care early and contact local health authorities in advance.
- Note any recent contact with others and travel details to provide to authorities who can trace and prevent spread of the disease.
- Stay up to date on COVID-19 developments issued by health authorities and follow their guidance.
Mask and glove usage
- Healthy individuals only need to wear a mask if taking care of a sick person.
- Wear a mask if you are coughing or sneezing.
- Masks are effective when used in combination with frequent hand cleaning.
- Do not touch the mask while wearing it. Clean hands if you touch the mask.
- Learn how to properly put on, remove and dispose of masks. Clean hands after disposing of the mask.
- Do not reuse single-use masks.
- Regularly washing bare hands is more effective against catching COVID-19 than wearing rubber gloves.
- The COVID-19 virus can still be picked up on rubber gloves and transmitted by touching your face.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Soo Kim is a Newsweek reporter based in London, U.K. She covers various lifestyle stories, specializing in travel and health.
Soo ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.








