A new strategy to stabilize carbon nitride photocatalyst for nitrogen reduction
Researchers from the Institute of Solid State Physics, Hefei Institutes of Physical Science developed a boron doped carbon nitride nanosheets with B-N-C coordination to stabilize surface exposed active nitrogen atoms and dramatically enhance photocatalytic ammonia synthesis performance. Their findings were published in Small.
It is well known that nitrogen (N2) accounts for ~78% in our atmospheric environment, which is essential element for almost all life forms including plants and animals on earth.
Up to now, the most mature technology of artificial synthetic ammonia (NH3) is the over century-old Haber-Bosch process, operated under drastic conditions due to the intrinsically inert characteristic of N2 molecules.
Currently, photocatalytic N2 reduction has been regarded as a promising means for sustainable NH3 synthesis at ambient conditions. As a class of metal-free polymer semiconductor photocatalyst, graphitic carbon nitride (g-C3N4) possesses many advantages, such as low cost, abundance, superior visible-light activity and high chemical/photochemical stability, exhibiting great potential for photocatalytic nitrogen reduction reaction (NRR).
However, several issues are still existent associated with the NRR using the g-C3N4-based photocatalyst. It is an important issue that exposed active nitrogen atoms (e.g., edge or amino N atoms) in g-C3N4 could participate in NH3 synthesis during the photocatalytic NRR.
Moreover, the pristine bulk g-C3N4 generally possesses relatively low specific surface area, which is adverse to the N2 adsorption and activation, as well as high recombination rate of photogenerated electrons and holes, resulting in its low photocatalytic efficiency.
Therefore, developing effective strategies to stabilize surface exposed active nitrogen atoms of g-C3N4, capable of improving the N2 adsorption/activation and visible-light utilization, simultaneously effectively inhibiting the recombination of photogenerated carriers in g-C3N4, is critically important and highly needed to achieve high-efficiency g-C3N4 based photocatalysts.
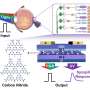
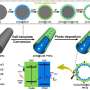
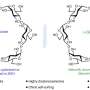
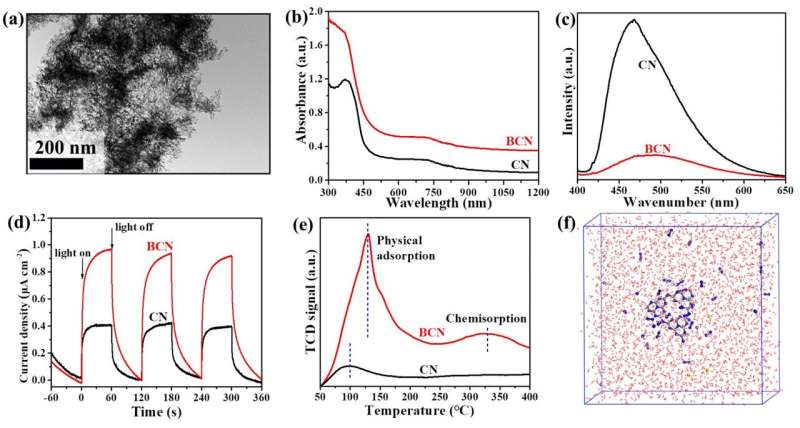
Herein, the team synthesized the porous B-doped g-C3N4 nanosheets (BCN) by a facile thermal treatment approach using dicyandiamide (DICY) and boron oxide (B2O3) as reactants. The formed B-N-C coordination in BCN not only effectively enhanced the visible-light harvesting and suppressed the recombination of photogenerated carriers in g-C3N4, but also acted as the catalytic active site for N2 adsorption, activation and hydrogenation.
The as-synthesized BCN exhibited high visible-light driven photocatalytic NRR activity, affording an NH3 yield rate of 313.9 μmol g–1 h–1, nearly 10 times of that for pristine g-C3N4.
More information: Weikang Wang et al. Formation of B-N-C Coordination to Stabilize the Exposed Active Nitrogen Atoms in g‐C 3 N 4 for Dramatically Enhanced Photocatalytic Ammonia Synthesis Performance, Small (2020). DOI: 10.1002/smll.201906880
Journal information: Small
Provided by Chinese Academy of Sciences