Review of POC Technologies
POC PCR
Diagnostic assays utilizing nucleic acid amplification testing (NAAT) are by far the most sensitive methods to detect pathogens in clinical samples. First reported in the literature by K Mullis et al. in 1986,[8] NAAT technologies have become standard clinical care for diagnosing a broad range of infectious diseases and play a growing role in cancer diagnostics. In particular, the standard of care for a person living with HIV in industrialized countries includes determining the HIV viral load, typically via NAAT, as an indication of treatment effectiveness. (A review of available HIV viral load testing methods is provided in: WHO/UNITAID: HIV/AIDS Diagnostics Technology Landscape 2014, 4th Edition[9]). However, in developing countries, due to lack of laboratory infrastructure and lack of highly trained laboratory staff, HIV viral load testing is restricted to a few central laboratories that have the necessary infrastructure and trained personnel to perform these more complex assays, thus limiting access to this important test for many patients that receive antiretroviral treatment.[10–12] This highlights the need for NAAT systems that have POC test attributes that meet the ASSURED criteria. Particularly important attributes in the developing world settings are low cost, ease-of-use, compatibility with intermittent electricity and a lack of clean water, and the ability to produce a result while the patient is still at the healthcare facility. Technology advances that reduce cycle time for nucleic acid amplification as well as approaches such as microfluidics that enable integrated sample preparation have paved the path to moving nucleic acid amplification-based testing out of high-infrastructure central labs into lower infrastructure healthcare settings. However, no POC PCR system available today has been demonstrated to meet all ASSURED criteria for the developing world, and only this year was a PCR test first able to achieve CLIA waiver in the USA.[13] NAAT continues to struggle with the cost, electrical power, and ease of use requirements for many low infrastructure settings, and widespread adoption in doctors' offices in industrialized nations is yet to be achieved. For these reasons, active development of POC PCR technology is ongoing.
Rapid Nucleic Acid Amplification Technologies. PCR, the most widely adopted nucleic acid amplification method, requires cycling the sample through three temperatures to double the number of DNA copies. With conventional PCR systems it can take up to 3 h to achieve the 20–35 cycles needed to detect low levels of DNA.[14] Miniaturization and microfluidics have played a key role in reducing PCR cycle times.[15] In particular, the introduction of methods whereby the sample is moved through stationary temperature zones as opposed to cycling a stationary sample through multiple temperatures has brought reaction times into the 20-min range.[16,17] Droplet-based microfluidic methods have emerged where DNA is loaded into nanodroplets together with PCR reagents, and these droplets are moved one by one on chip between temperature zones, enabling three-times faster PCR reactions than conventional methods.[18] In a more recent study, sub-100 s reaction for 35-cycle amplification was achieved by improving heat transfer and optimizing polymerase chemistry.[19]
While significant progress has been made adapting thermocycling-based NAAT diagnostics for low-infrastructure environments, challenges such as a relatively high cost per test, the requirement for uninterrupted line power and the need for skilled operators with access to continuous training to maintain proficiency have limited adoption of PCR systems. Perhaps the most illustrative example to highlight the current status of POC PCR testing in low resource settings is the Cepheid (CA, USA) GeneXpert®, which has been heavily subsidized and broadly deployed as part of the global fight against tuberculosis.[20] The Cepheid Xpert® MTB/Rif has been widely distributed in South Africa and has been reported to be 'feasible at the POC when done by nonspecialized personnel'.[21] However, the technology has not had the desired impact, both because it requires more complex infrastructure and higher levels of training than are accessible in many parts of South Africa and because the healthcare system does not always provide timely treatment initiation after positive diagnosis.[21–25]
Isothermal amplification methods that replicate nucleic acids at a single temperature and thus eliminate the need for thermal cycling have also emerged.[26] Loop-mediated isothermal amplification (LAMP), which was introduced in 2000,[27] is the most widely adopted isothermal amplification method and has been explored for near-patient applications including TB and malaria.[28,29] While the LAMP assay satisfies some of the ASSURED criteria in that it is fast, can be read out visually, and only requires minimal power to operate a heat block, a WHO Expert Group found that further improvements in ease of use are needed to make the assay robust enough to be used in settings that lack uninterrupted power supply, experience high temperatures, and humidity and lack skilled operators.[30]
It is also important to note that isothermal amplification methods can be challenging to make quantitative for applications such as HIV viral load assays. Nucleic-acid sequence-based amplification together with molecular beacons for detection[31] is an example of a quantitative isothermal amplification method that has been applied to HIV viral load testing but not at the POC since it still requires a lab infrastructure.[32]
Sample Preparation. Nucleic acid isolation and purification from patient samples is a time-consuming and cumbersome process. This can be minimized in high-infrastructure clinical labs with automated liquid handling systems that feed high-throughput PCR systems. In general, though, simplifying and integrating sample preparation with POC PCR assays has presented one of the major challenges for achieving true 'sample-in, result-out' POC PCR systems. Several approaches have emerged that rely heavily on miniaturization and microfluidics to achieve this goal.[33] One such system that combines sample preparation with amplification and detection is the 'simple amplification-based assay' (SAMBA) to differentiate between patients with viral loads above or below 1000 copies/ml within 90 min.[34] The assay utilizes a disposable cartridge that is preloaded with all necessary reagents. RNA is extracted from plasma that was added to the cartridge using a sample preparation instrument. Nucleic acid hybridization on a dipstick membrane and a signal amplification technology are used to visually detect the isothermal amplification product.[35] While methods such as SAMBA offer promising routes to streamlining sample preparation workflow, NAAT methods still struggle to meet the requirements of POC testing, particularly in the developing world, and are most likely to be found in select laboratories in urban cities that are often far from outbreaks.
Immunoassays
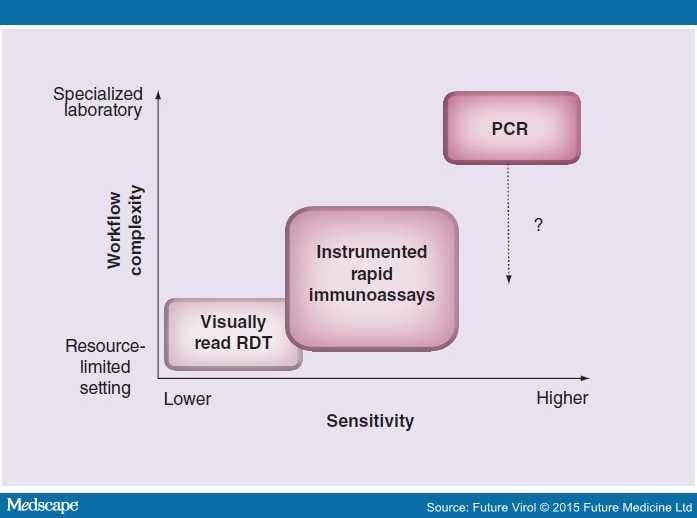
In contrast to NAAT methods, which provide superior analytical sensitivity at the cost of a more complex workflow, immunoassays offer inherent simplicity that is ideally suited for POC testing (see Figure 1). Thus, despite limitations, immunoassays continue to be a mainstay of POC testing.[36] POC immunoassays for infectious disease applications generally fall into one of two distinct categories: serological assays which assess an individual's immune response to an infectious agent or direct antigen testing as a diagnostic method for detecting the presence of an infectious agent in a patient-collected specimen. If an application dictates, a single POC immunoassay assessing both presence of an infectious agent and characterization of an individual's immune response to that agent can be developed.[37,38] Typical specimen types for POC immunoassay testing include bodily fluids such as blood, serum, plasma, urine or salivary secretions as well as swabs or liquid wash specimens collected from any of a number of different body sites. In low infrastructure environments, specimen collection may be particularly challenging because of the lack of trained phlebotomists or absence of private spaces (e.g., for collection of cervical swabs). Regardless of the specimen type used, it is critical that specimen collection be properly conducted in order to maximize the performance of the POC test.
Figure 1.
Point-of-care viral diagnostics must often trade off performance and ease of use. For example, while PCR represents state-of-the-art analytical sensitivity, it is still a costly and complex test method, and it is uncertain whether the workflow can be simplified to make this method appropriate for the most resource-limited settings. Visually read RDTs incorporate extremely simple workflows but can result in suboptimal analytical and clinical sensitivity.
RDT: Rapid diagnostic test.
Serology assays can be used for screening applications such as for HIV and Hepatitis C detection in blood supplies due to the high sensitivity and specificity associated with this testing format. In the case of POC clinical diagnostics, serology assays can be configured to detect the presence of IgM antibodies as an indicator of new or active infection. Alternatively, they can detect IgG antibodies as a convalescent phase indicator, providing information about past exposure to the infectious agent or assessing active immune status as a measure of an individual's ability to respond to a specific infectious agent. Tests are also available which can assess the presence of agent-specific IgM and IgG simultaneously. Serology assays typically use serum or plasma as a specimen type, but a significant number of tests using whole blood specimens have been developed, which has helped drive their use outside of the laboratory environment. POC serology assays are used worldwide for HIV diagnosis and have enabled testing in environments and geographies where skilled labor and laboratory facilities are virtually nonexistent.[39] This category of assays is also widely used for other chronic disease indications such as infection with blood-borne hepatitis viruses, CMV, HSV, or bacterial pathogens such as Mycoplasma pneumoniae, Borrelia species (Lyme disease) or Treponema pallidum (syphilis). Often, the impetus for performing a POC test is to generate a result for which there is an actionable response that can be administered by the healthcare provider. Given the lag between initial infection and development of an immune response to the causative agent, serology tests may not be appropriate for diagnosis of acute primary infection. However, these tests may inform treatment decisions. For example, in the case of Dengue, serology tests can distinguish between primary and secondary infections, so that patients with secondary infections who are at a higher risk for hemorrhagic fever can be clinically followed more closely.
For acute disease diagnosis, direct antigen testing is often preferred.[40,41] Unlike serology assays, direct antigen tests are used almost exclusively in a confirmatory mode and therefore indications for use for these tests rarely, if ever, include screening applications. These assays are configured using antibodies or other binding reagents that target specific molecules unique to the suspected infectious agent, hence the confirmatory indications for which these tests are employed. Typical target molecules are cell- or virus-associated surface proteins, organism-specific lipopolysaccharides, nucleic acid packaging proteins or other multiple copy antigens specific to the given infectious agent. Since these are antigen tests, they are not compatible with amplification techniques such as are used in PCR. Therefore, choice of an appropriate target molecule is critical, with the ideal target displaying both the highest level of specificity for the organism of interest and the highest possible copy number per organism to be detected.
Initial direct antigen POC immunoassays were derivatives of standard microtiter plate-based ELISAs and were configured primarily in flow-through or agglutination formats which required multiple user steps, restricting their use to skilled, laboratory-based personnel. The emergence of immunoassays in the one-step, lateral flow format in the 1980s eliminated many of these workflow concerns and facilitated the dissemination of these tests outside the laboratory environment. Typical lateral flow POC immunoassays require no, or very simple, specimen preparation, and the sample is added directly to the test strip or test device.[36] Most infectious disease POC assays are configured in a sandwich immunoassay format (requiring two antibodies) where one target-specific antibody is conjugated to a mobile, detector particle resident at one end of the test strip/device, and a second target-specific antibody which is deposited in a defined test zone on the strip/device. The sample is added in proximity to the dried detector conjugate in order to reconstitute it, and the mixture flows up/across the test strip, generating a visual signal in the defined test position of the strip if the target antigen of interest is present. The signal results from accumulation of detector particle–target antigen complexes in the test zone by virtue of secondary capture attributed to the test zone antibody. If the suspected infectious agent is not present, or if the level of the target protein is below the limit of detection for the assay, no visual signal is generated. Many types of detector labels can be used for these assay including colloidal metal particles, colored latex particles, fluorescent particles or directly labeled mobile phase antibodies. Typical time-to-result for this category of tests is 5–15 min.
Given the simplicity and rapid time to result for these tests, they are routinely used in nonlaboratory testing environments. Although the assay workflow enables the participation of test operators with little or no laboratory skills, proper specimen collection is crucial and therefore should be carried out by a trained healthcare professional. When used appropriately, these tests can provide valuable information to help influence key therapeutic or treatment decisions by the healthcare provider.
Emerging Technologies
Beyond the more established diagnostic approaches outlined above, alternatives based on emerging technologies are being pursued. Diagnostics based on these emerging technologies have reached varying levels of maturity due, among other factors, to the inherent challenges of translating established approaches to new platforms or developing new methodologies altogether. A common obstacle to translating both immunoassay and molecular-based tests to the POC, and the focus of many emerging technologies, is sample handling and preparation.[42–44] As mentioned above, the need for robust, low-cost and integrated sample preparation has spurred significant efforts in microfluidics, since these types of approaches offer the potential to enable a so-called 'lab on a chip' that integrates complex assays for low sample volumes into a simplified workflow at a low cost. If fully realized, the 'lab on a chip' concept would enable any test to move to a POC format. However, in spite of over two decades of development, microfluidics has not yet brought about the revolution in biology and diagnostics that many expected.[45–47] Integrating multiple laboratory processes into a small disposable device can be complex, and the challenge of doing so cost-effectively has limited the technology's commercial applications to date. There are certainly commercial applications of microfluidics, as described above, and as also found in viral detection tests such as, for example, Verigene® Respiratory Virus Plus (Nanosphere, IL, USA), -Xpert® Flu RT-PCR (Cepheid), and Simplexa™ RT-PCR (Focus Diagnostics, CA, USA). However, the applications are still relatively few and typically require supporting instrumentation, challenging the idea that the lab is on the chip.
While conventional 'lab on a chip' approaches continue to be pursued, new approaches are seeking alternative ways of managing complex fluid processes on-chip and lowering the cost of microfluidic devices. Electrowetting-based digital microfluidics simplifies the integration of on-chip processes by eliminating the need for pumps and valves,[48,49] which can be complex and expensive components in conventional designs. At the same time, the approach provides programmable sample manipulation, reducing the need for a customized microfluidic design for each application. Paper-based microfluidics provides an extremely low-cost route to microfluidic-based diagnostics, making it ideal for both developed and developing world applications.[50,51] The processes with which it is compatible are currently more limited than 'lab on a chip' solutions, but the levels of complexity and integration it offers continue to grow.[44,52]
Complementing the work in microfluidics are efforts by a number of groups to improve the analytical sensitivity of virus detection assays, particularly for nonmolecular assays (i.e., for assays without the inherent complexity of molecular amplification). For example, imaging-based approaches have shown detection down to the single virion level. One example is a cell-phone-based imaging system that detects individual fluorescently labeled virions deposited on a coverslip.[53] A second example called IRIS (interferometric reflectance imaging sensor) provides detection in a label-free format by imaging the optical interference between light scattered from viruses and a reflected reference wave.[54] Other optical detection approaches have also been reported to show sensitive detection of viruses. These include the use of engineered substrates for label-free SERS (surface-enhanced Raman spectroscopy)-based detection,[55] optical microring resonators based on semiconductor technology[56] and guided optical waves in silica microspheres.[57] While methods that resolve very few, or even individual, virions would seem to offer a definitive advantage over competing techniques, it is not yet clear that the ability to detect individual bound viruses translates into lower limits of detection in a clinical diagnostic test. In such applications, the ability to deliver the target to the detector surface, the limited affinity of recognition molecules such as antibodies and nonspecific binding effects are likely to be the determining factors in sensitivity. Nonetheless, if effective, very sensitive detection approaches may shift the focus from the detection system to other factors driving analytical sensitivity.
One emerging technology that offers promise for simplifying the collection aspect of sample handling is the analysis of volatile organic compounds (VOCs) in patients' breath to diagnose disease. The approach provides great appeal for its potential to provide noninvasive analysis and monitoring of disease state in near-real time.[58] Much of the work to date has been directed toward detection of cancer and bacterial respiratory infections,[58,59] although it has recently been applied to analysis of VOCs produced by influenza virus in culture.[60] The development of VOC analysis for routine clinical use and extension to POC has been slow, due largely to the challenges of identifying sensitive and disease-specific biomarkers in VOCs; the relatively weak concentrations of these biomarkers (often parts-per-billion to parts-per-trillion); generating informative analysis of the complex measured signals; lack of standardization for sample collection and pre-concentration; and high costs of instrumentation.[58,59] As progress on these fronts continues, VOC analysis may provide an effective and appealing POC viral diagnostic.
As others have pointed out, there are numerous hurdles between demonstration of a new technology and its routine clinical use.[61] Many of the technologies here, while demonstrating potential, have not yet shown the clinical sample performance, advantage over alternative techniques, level of integration (with sample preparation, readout, or analysis), application suitability (including workflow and cost) and/or acceptance (including regulatory and end-user) necessary to become the standard of care. In following sections, we review key considerations in translating established diagnostic techniques to the POC using three specific examples.
Future Virology. 2015;10(3):313-328. © 2015 Future Medicine Ltd.