Abstract and Introduction
Background: The proliferation of inhaler devices has resulted in a confusing number of choices for clinicians who are selecting a delivery device for aerosol therapy. There are advantages and disadvantages associated with each device category. Evidence-based guidelines for the selection of the appropriate aerosol delivery device in specific clinical settings are needed.
Aim: (1) To compare the efficacy and adverse effects of treatment using nebulizers vs pressurized metered-dose inhalers (MDIs) with or without a spacer/holding chamber vs dry powder inhalers (DPIs) as delivery systems for β-agonists, anticholinergic agents, and corticosteroids for several commonly encountered clinical settings and patient populations, and (2) to provide recommendationsto clinicians to aid them in selecting a particular aerosol delivery device for their patients.
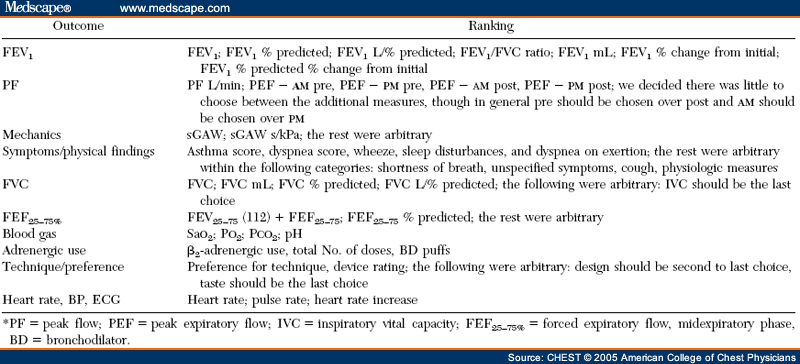
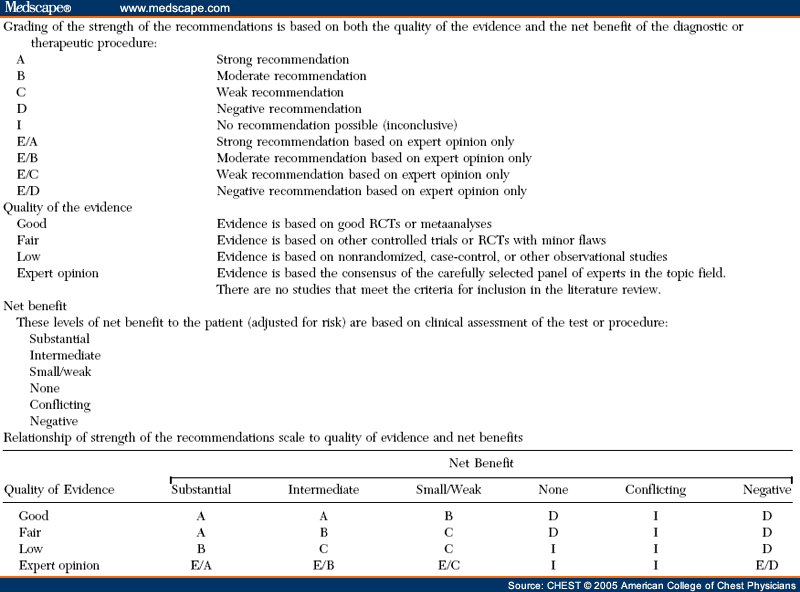
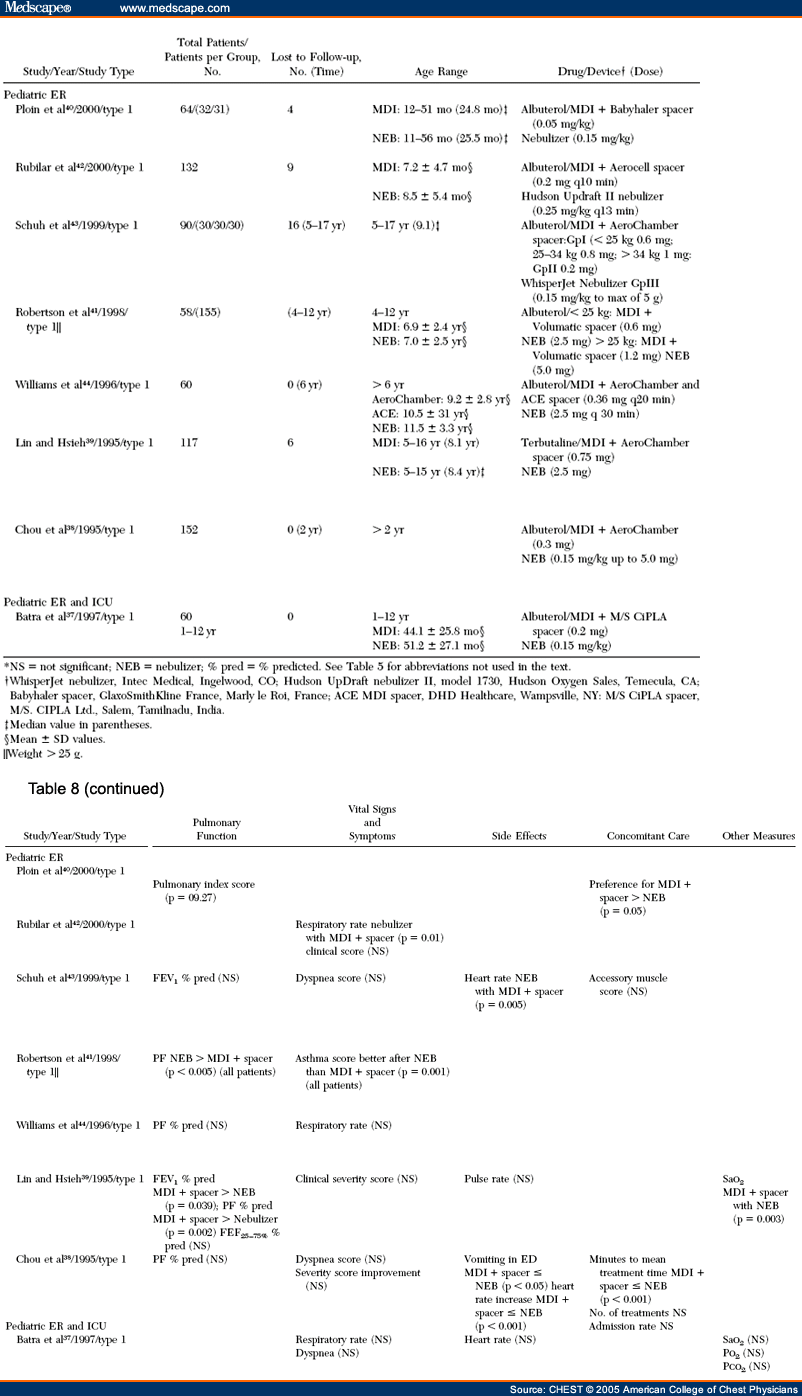
Methods: A systematic review of pertinent randomized, controlled clinical trials (RCTs) was undertaken using MEDLINE, EmBase, and the Cochrane Library databases. A broad search strategy was chosen, combining terms related to aerosol devices or drugs with the diseases of interest in various patient groups and clinical settings. Only RCTs in which the same drug was administered with different devices were included. RCTs (394 trials) assessing inhaled corticosteroid,β2-agonist, and anticholinergic agents delivered by an MDI, an MDI with a spacer/ holding chamber, a nebulizer, or a DPI were identified for the years 1982 to 2001. A total of 254 outcomes were tabulated. Of the 131 studies that met the eligibility criteria, only 59 (primarily those that tested β2-agonists) proved to have useable data.
Results: None of the pooled metaanalyses showed a significant difference between devices in any efficacy outcome in any patient group for each of the clinical settings that was investigated. The adverse effects that were reported were minimal and were related to the increased drug dose that was delivered. Each of the delivery devices provided similar outcomes in patients using the correct technique for inhalation.
Conclusions: Devices used for the delivery of bronchodilators and steroids can be equally efficacious. When selecting an aerosol delivery device for patients with asthma and COPD, the following should be considered: device/drug availability; clinical setting; patient age and the ability to use the selected device correctly; device use with multiple medications; cost and reimbursement; drug administration time; convenience in both outpatient and inpatient settings; and physician and patient preference.
The use of inhaled aerosol medications for the treatment of pulmonary diseases, which became well-established in the last half of the 20th century, has advantages over oral and parenteral routes of delivery. The use of inhaled aerosols allows selective treatment of the lungs directly by achieving high drug concentrations in the airway while reducing systemic adverse effects by minimizing systemic drug levels.[1] Inhaled β2-agonist bronchodilators produce a more rapid onset of action than oral delivery. Some drugs are only active with aerosol delivery ( eg, for asthma patients, cromolyn and ciclesonide; for cystic fibrosis patients, dornase alfa). Aerosol drug delivery is painless and often convenient. For these reasons, the National Asthma Education and Prevention Program guidelines[2] favor aerosol inhalation over the oral route or parenteral ( ie, subcutaneous, IM, or IV) route. Similarly, the National Heart, Lung, and Blood Institute/World Health Organization Global Initiative for Chronic Obstructive Lung Disease recommended that bronchodilator medications are central to symptom management in COPD patients and that inhaled therapy is preferred.[3]
There are also disadvantages to aerosol drug therapy.One of the most important disadvantages is that specific inhalation techniques are necessary for the proper use of each of the available types of inhaler device. A less than optimal technique can result in decreased drug delivery and potentially reduced efficacy.[4,5] Improper inhaler technique is common among patients.[6–8] The proliferation of inhalation devices that are available for patients has resulted in a confusing number of choices for the health-care provider and in confusion for both clinicians and patients trying to use these devices correctly. Several studies have demonstrated lack of physician, nurse, and respiratory therapist knowledge of device[9–13] Inhaler devices are less convenient than oral drug administration insofar as the time required for drug administration may be longer and some patients may find the device less portable. This is particularly true for conventional compressed-air nebulizers, the oldest of the currently used types of aerosol delivery devices.
Device manufacturers have long been aware of the importance of portability and ease of use with aerosol delivery devices. As a result, these devices have evolved over time. From the 19th century until 1956, compressed-air nebulizers (also called jet nebulizers) were the only devices that were in common clinical use for the administration of inhaled aerosol drugs. In 1955, the pressurized metered-dose inhaler (MDI) was developed at Riker Laboratories (now 3M Pharmaceuticals; St. Paul, MN).[14] Ultrasonic nebulizers, which utilize high-frequency acoustical energy for the aerosolization of a liquid, were introduced in the 1960s.[15,16] In 1971, Bell and colleagues[17] introduced the first dry powder inhaler (DPI), known as the Spinhaler , for the inhalation of cromolyn sodium. This and subsequent DPIs have been "breath-actuated," providing drug only when demanded by patient inhalation, thus avoiding a common error with MDI use, the improper timing of inhaler actuation. Breath-actuated MDI devices ( eg, the Autohaler; 3M Pharmaceuticals) are also triggered by patient inhalation to release the drug on demand.
Investigators developed open-tube spacer devices, intended for use with MDIs, in the late 1970s.[18–20] The addition of a one-way valve (holding chamber)[18] or blind reservoir ( ie, reverse-flow spacer)[21,22] allowed the aerosol delivered by the MDI to be contained in the spacer for a finite period of time, thereby circumventing the need for the coordinated actuation of the MDI with inhalation. Other spacer/ holding chamber designs followed, and today there are several devices that vary in design, shape, size, and assembly. The design of MDIs changed little between 1956 and the 1980s. However, the 1987 Montreal protocol mandated the phaseout of the use of chlorofluorocarbons (CFCs) as propellants in all MDIs. This resulted in a redesign of MDIs in the 1990s, utilizing hydrofluoroalkane propellants.[22] Some of these formulations produce aerosols with different characteristics that behave differently in patients than their predecessors.[23]
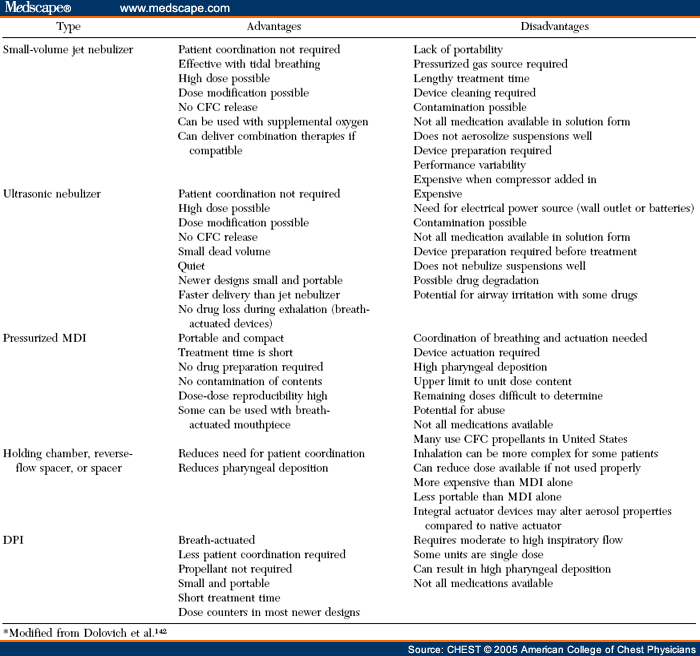
Each type of aerosol device has its own advantages and disadvantages ( Table 1 ). Nebulizer/compressor systems require minimal patient cooperation and coordination, but are cumbersome and time-consuming to use. Matching nebulizers with associated air compressors is necessary to assure optimal efficiency of drug delivery. MDIs are quicker to use and highly portable, but require the most patient training to ensure coordination for proper use. Up to 70% of patients fail to use them properly. The improper timing of MDI actuation with breath initiation is a common problem.[7] DPIs are easier to use than MDIs because they are breath-actuated, but require a relatively rapid rate of inhalation in order to provide the energy necessary for drug aerosolization. Younger patients and patients in acute distress may not be able to generate the necessary flow rates.[2,24] Breath-actuated MDIs are also easier to use, but are currently available in the United States for only a single drug ( ie, the β2-agonist pirbuterol). Holding chambers used with MDIs remove the necessity of careful timing between inhalation and MDI actuation. However, they are more bulky to carry than the MDI by itself or a DPI. The improper use of holding chambers ( eg, placing multiple puffs in the chamber before inhalation or waiting too long between MDI actuation and inhalation) can actually reduce drug delivery to the lungs.
Several factors can guide clinicians on the choice of a device for a specific patient. One factor is the age of the subject ( Table 2 ).[2] Another factor is the availability of the drug formulation, as not all drugs are available in each type of aerosol delivery device. The clinical setting ( eg, outpatient, emergency department (ED), hospitalized inpatient, or intensive care setting) and the disease being treated ( eg, COPD vs asthma) also influence the choice of aerosol device.
Several systematic reviews and metaanalyses related to the selection of an aerosol delivery device have been published. In a metaanalysis, Turner et al[25] concluded that bronchodilator delivery by means of nebulizer or MDI is equivalent in the treatment of adults with acute airflow obstruction. A systematic review by Amirav and Newhouse[26] compared MDIs with accessory devices to nebulizers in children with acute asthma. While their results showed no differences between the types of delivery systems, it was concluded that the MDI with an accessory device ( ie, a spacer or holding chamber) should be considered the preferred mode of aerosol delivery. A systematic review[27] of the management of acute exacerbations of COPD concluded that there is insufficient evidence that either an MDI or a nebulizer is superior. Cates et al[28] and Cates,[29] in systematic reviews of spacers and holding chambers vs nebulizers for β2-agonist treatment of acute asthma, concluded that an MDI with a holding chamber produces outcomes that are at least equivalent to those achieved with the use of a nebulizer. Several systematic reviews[30–32] have compared MDIs to DPIs and have concluded that there is no evidence that either device is superior to the other for bronchodilator therapy.
While systematic reviews provide key evidence summaries, they do not present specific recommendations for practice. The reasons for this include a focus on restricted populations and outcomes, and the lack of a process to ensure recommendations reflect patients' values and preferences.[33] However, clinicians require information and guidance concerning the best estimates of benefits and risks of alternatives, and concerning the explicit tradeoffs between these benefits and risks, or, in other words, evidence-based guidelines. Therefore, despite the availability of the above systematic reviews, we believe that evidence-based guidelines are still needed. Consequently, the intent of this project was to assess the available scientific evidence addressing the question of whether device selection affects efficacy and the adverse effects of treatment. Therefore, we set out to systematically review relevant evidence from randomized, placebo-controlled clinical trials and to provide general recommendations based on the tradeoffs that this evidence provides. Our recommendations relate to issues that clinicians should consider in selecting a particular therapeutic aerosol delivery device for their patients in each of several commonly encountered clinical settings.
CHEST. 2005;127(1):335-371. © 2005 American College of Chest Physicians
Cite this: Device Selection and Outcomes of Aerosol Therapy - Medscape - Jan 01, 2005.














Comments