Optical Coherence Tomography
OCT is based on the reflection of light.[62] However, owing to the speed of light being so much faster than sound (approximately 872,000 times - 299,792,458 m/s compared with 344 m/s), interference is used rather than the time between light-pulse emission and detection of reflections. Lateral resolution of the image is a function of the optics of the device. Axial resolution, conversely, is equivalent to the coherence length of the light source. Conventional interferometry (laser) has long coherence length (in the order of a meter), so to image the eye at high resolution, broadband (covering a range of frequencies) light sources are used to shorten the coherence to micrometers. These broadband light sources are typically superluminescent diodes (bright-light-emitting diodes) and lasers with extremely short pulses (femtosecond lasers). An interference pattern occurs if light from the reference arm (reflected from a mirror) and the measurement arm (reflected from ocular surfaces) travel the "same" optical distance (i.e., a difference of less than a coherence length) before being recombined. Any light that is outside the short coherence length will not contribute to the coherence pattern. In time-domain OCT, a reflectivity profile (A-scan) is built by the reference mirror physically scanning over the coherence length. This feature of time domain OCT limits the potential resolution and clarity of image. High-reflectivity surfaces and media will cause greater interference when mixed with the reference light than low-reflectivity media. A cross-sectional tomograph (B-scan) may be achieved by laterally combining a series of these axial depth scans (A-scan).
Spectral OCT negates the requirement to oscillate the reference mirror to create broadband interference by spectrally separating the detectors, either by encoding the optical frequency in time with a spectrally scanning or "swept" source or with a dispersive detector, such as a grating and a linear detector array.[63,64] The depth scan can be calculated by a Fourier-transform from the acquired spectra without movement of the reference arm. The spectral bandwidth sets the axial resolution and resolution as low as 3-5 µm has been achieved.[39] The imaging speed is improved and there are potential improvements in the signal-to-noise ratio proportional to the number of detector (silicon imaging chip) detection elements (pixels). The fall off in signal-to-noise ratio with penetration depth in spectral dispersive OCT is overcome by swept source OCT; however, it has been suggested that there are nonlinearities with wavelength, especially at high scanning frequencies.[65]
As with any image reconstruction technique where a wave, such as light or sound, passes through curved media of differing refractive indices, the image needs to be compensated for these distortions to allow spatially accurate presentation and measurement. If the cornea is imaged, this can be detected using edge detection algorithms and fitted to correct the image of the deeper surfaces. However, the corneal surfaces are usually outside the field of view if the crystalline lens or intraocular lens is imaged, and none of the optical surfaces are analyzed when observing the retina, so image distortions can only be overcome by successive surface analysis.
Clinically, the OCT allows in vivo cross-sectional imaging of tissue, a major advance over techniques such as photography and scanning laser ophthalmoscopy. The OCT has been used extensively in imaging the retina and anterior eye, although a longer-wavelength illumination source (typically 1310 nm rather than 840 nm) is required for imaging the anterior eye structures due to penetration and tissue absorption light characteristics. When applied to the retina, OCT has principally been used to examine macular changes in conditions, such as diabetes, macular degeneration and hole formation.[66,67,68] OCT measures of RNFL thickness are a good independent predictor of the development of glaucomatous change in glaucoma suspects, even when adjusting for stereophotograph assessment, age, intraocular pressure, central corneal thickness and automated perimetry pattern standard deviation.[69,70] OCT can also be applied to the noninvasive, in vivo assessment of retinal blood flow.[71]
Anterior segment OCT (Figure 6) has been used in the evaluation of contact lenses,[72] corneal thickness,[73] corneal grafts,[74] corneal implants,[75] laser refractive surgery,[76] intraocular lens tilt, centration and corneal clearance,[77] intraocular lens sizing[78] and posterior subcapsular opacification.[79] In angle closure glaucoma, OCT is sensitive compared with gonioscopy and identifying closed angles more frequently than gonioscopy.[80] Anterior segment OCT can also provide morphology not visible with slit-lamp biomicroscopty, such as in the evaluation of trabeculectomy blebs.[81] Another application of anterior eye OCT of particular importance is in corneal wound construction and healing.[82] Recent studies have suggested that clear corneal cataract incisions are linked with an increased incidence of endophthalmitis, proposed to be due to incomplete wound self-sealing, although there is much debate.[83,84,85,86,87] Cross-sectional images of the cornea following surgery show that the wound architecture is more arched than previously proposed, with stromal swelling proposed as part of the sealing mechanism.[82]
Optical coherence tomography image of the anterior chamber of a pseudophakic eye.
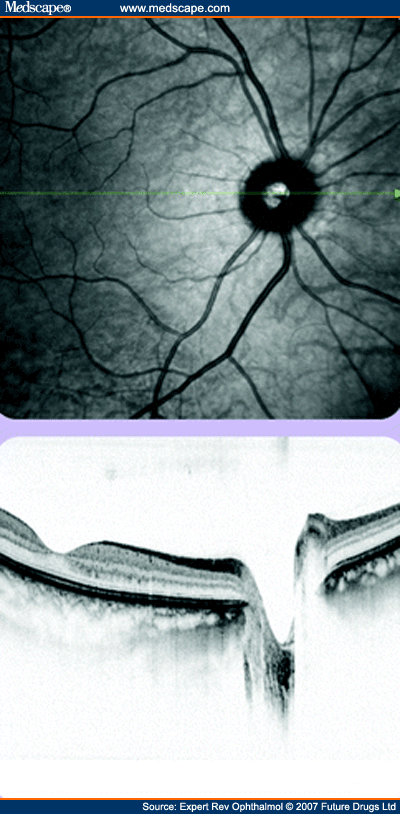
As with other imaging techniques, OCT has been combined with AO to enhance the image quality and resolution. This is particularly important in patients with cataract, in whom OCT images are degraded significantly.[88] Bigelow and colleagues have developed a compact spectral domain OCT combined with a Hartmann-Shack wavefront sensor and microelectromechanical systems-based deformable mirror, which is able to compensate the captured image at a rate of 15 Hz, increasing the signal by up to 8 dB and decreasing the lateral resolution to less than 10 µm.[39] Roorda and coworkers have recently demonstrated visualization of individual RPE cells using AO OCT.[89] 3D images of the retina can be constructed from multiple spectral domain OCT sections that are spatially registered. These constructed in vivo images have been compared with histological examination in animal retinas and shown to correspond well.[90] Quantitative measures, such as volume measures, can be determined and followed longitudinally, enhancing the study of ocular disease. Dual-channel systems have been developed with OCT and scanning laser ophthalmoscopy images to enable clinicians to visualize retinal sections side by side with standard fundus images or angiograms to aid localization (Figure 7).[91]
Scanning laser ophthalmoscopy image of the fundus to allow localization with the combined optical coherence tomography section, in this case through the macular and optic disc.
Expert Rev Ophthalmol. 2007;2(5):755-767. © 2007 Future Drugs Ltd.
Cite this: Advances in Ocular Imaging - Medscape - Oct 01, 2007.









Comments