Treating cancer with sperm
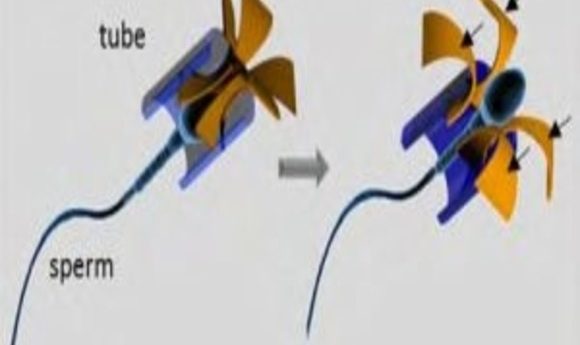
Scientists hope to take advantage of the natural swimming abilities of sperm to target tumor cells.
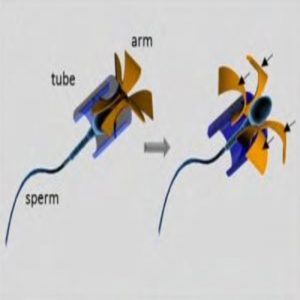
Illustration of the sperm-hybrid micromotor and the sperm release process (1).
For decades, researchers have sought out ways to target cancer cells for treatment while leaving healthy neighboring cells untouched—and more importantly, the immune system functioning. To this end, scientists have repurposed macrophages, packing them full of drugs and setting them loose to hunt down and destroy tumor cells without affecting other cells. They have also enlisted disease-causing bacteria to colonize and destroy tumors by genetically altering them to produce anti-cancer drugs or by relying on their innate pathogenic powers against cancer cells and ability to call in the immune system.
Recently, Oliver Schmidt from the Leibniz Institute for Solid State and Materials Research in Dresden, Germany proposed using a new cell type for specifically targeting tumors, one whose only purpose is to travel through an inhospitable environment seeking out one unique cell among the millions it encounters: sperm. Of course with its solitary goal being to reach the egg, a sperm cell needs some guidance to hunt down a tumor cell instead.
“The idea to use sperm cells coupled to a synthetic component for a better external control fascinated us,” recalled Mariana Medina-Sanchez, who co-authored a paper with Schmidt reporting the development and testing of their so called “spermbot” (1). “We found that it can also work as a potential drug carrier to treat diseases in the female reproductive tract, as this is the environment sperms are naturally adapted to swim in.”
Dip a Toe
Schmidt is interested in developing microrobots for environmental and biomedical applications. In 2013, he and his colleagues Veronika Magdanz and Samuel Sanchez designed the first sperm-flagella driven microbiorobot (2), where they encased bovine sperm inside a magnetic microtube. The researchers then guided the direction of swimming while the sperm propelled the device forward, suggesting that these bots might be useful for assisted reproduction in cases of low sperm count. The team later developed a spiral-shaped metal-coated polymer motor to serve as a flagella prosthesis for sperm with low motility. Guided by magnets, this bot scooped up stranded sperm and delivered them wherever the researchers directed their paths (3).
In addition to swimming—and now propelling microbots—sperm possess several other qualities that suggested they might have a lot to offer even beyond the field of assisted reproduction. Previous research showed that sperm could absorb large quantities of protein and hydrophilic drugs and that their compact membrane system protects those drugs from dilution by body fluids and detection by the immune system and degradation enzymes. Sperm not only fuse with eggs during fertilization, but they can also fuse with somatic cells. And they are fine-tuned for swimming through the inhospitable environment of the female reproductive tract. Schmidt and his team began to wonder if spermbots might provide the ideal means of precisely targeted drug delivery for gynecological cancers.
“In principle, any other kind of cell containing drugs could be transported by a synthetic swimmer to the region of interest using external sources such as magnetic field, ultrasound, or light,” Medina-Sanchez explained. “We particularly chose the sperm cell because of its ability to swim through complex environments and its relatively low but sufficient lifetime.”
Make a Splash
Schmidt and his team needed a way to redirect the sperm towards tumor cells, so they decided to design a harness they could guide using magnets. This harness required greater flexibility than the microtube they used in their first sperm-propelled microbot. In this case, the sperm needed to enter the harness, travel to the tumor, and then escape it in order to fuse with tumor cells and deliver the drug cargo.
“The microstructure that we used to guide and release drug-loaded sperms was made by 3D laser lithography—the desired shapes were printed out of liquid polymer,” Medina-Sanchez said. “Then such microstructures were coated with a thin layer of iron to provide them with magnetic properties that allow us to control their direction using an external magnetic field.”
The team designed the harness with a tubular body to easily encase the head of the sperm and four flexible arched arms that protrude from one end of the tube. When the arms hit an object, such as a cluster of tumor cells or a culture plate wall, they bend, enlarging the harness opening to allow the sperm to break free.
To test their new design, the team turned to bovine sperm since they are similar in shape to human sperm. Once securely inside the harness, the sperm tails beat freely enough to provide powerful propulsion, although the extra weight and fluid drag on the harness decreased swimming speeds. The harness didn’t influence the path of swimming, however, since it rotated with the helical motion of the sperm.
In further testing, the researchers learned that they could direct the sperm using an external magnet, but even though the arms of the harness flexed as expected when they hit a barrier, the sperm often got stuck inside. Schmidt and his team solved this problem by simply coating the inside of the harnesses with a sperm-repelling agent.
Sink or Swim
With the spermbot functioning, the team next turned to the question of drug delivery. They soaked sperm in a solution of doxorubicin (DOX), a chemotherapeutic agent commonly used to treat cancer including gynecological cancers. Fluorescence imaging showed that the sperm efficiently absorbed DOX in their heads and midregions, mainly concentrating it within the cytoplasm and nucleus. After 96 hours, very little of the drug leaked from the sperm, and the sperm themselves were not harmed, nor did they degrade the drug due to their incomplete metabolic systems.
Schmidt’s team introduced their drug-laden spermbots to cultures of adherent and spheroid cervical cancer cells. After 72 hours, the density of cancer cells cultured with the drugged spermbots decreased by half compared to groups treated with un-drugged sperm or to other control cultures. Cells remaining in the plate rounded up and exhibited blebbing, indicating they were undergoing apoptosis. And the cancer cells showed that they had taken in the drug.
Interestingly, unloaded sperm killed cancer cells in long-term spheroid cultures, possibly due to penetration and disaggregation of the culture over long culture periods. In contrast, diluting drugs in the culture media led to cell death in equal rates as those seen with drugged spermbots, but only in the first 2 days of culture. After that, spermbots continued killing the cells, while diluted drugs did not; the researchers suggested that this shows one advantage of containing the drug within the sperm rather than allowing it to diffuse where it can be diluted by body fluids.
“The combination of sperm as motor and drug delivery shuttle, together with a nanostructure made with a 3-D printer that allows guidance of the shuttle is very elegant!” said Sebastian Felgner from the Helmholtz Center for Infection Research in Braunschweig, Germany, who is currently developing bacteria to precisely target cancer cells and was not involved in this study. “However, all experiments were carried out in cell culture. It remains to be shown how the construct performs under physiological conditions. Nevertheless, it’s an idea that deserves to be followed up.”
Just Keep Swimming
Medina-Sanchez, Schmidt, and their colleagues published an article describing the spermbot design and early testing on the preprint server Arxiv. “In general, the feedback has been positive. We have talked with people in the field of fertilization and oncology, and they consider this platform as a promising alternative to bring drugs or substances to treat diseases in the female reproductive tract, not only cancer, but also some other female fertility problems,” Medina-Sanchez said.
The team is now focusing their next steps in developing the spermbot. In particular, they would like to modify the harness such that they can trigger release of the drug-loaded sperm at particular times and places, and they also want to improve the guidance system so that they can direct multiple sperm cells towards a tumor at once—and then there is also the question of the drug delivery mechanism.
“The mechanism of drug transfer from the sperm to the tumor cells is not fully understood yet and needs exhaustive studies,” Medina-Sanchez said. “We know that the sperm has the ability to fuse with somatic cells, and if this fusion happens, we believe that the drug can be transferred from cell to cell.”