Abstract
In a large cohort study of the German Neonatal Network (GNN) we aimed to evaluate whether less invasive surfactant administration (LISA) strategy is associated with complications of preterm birth. Within the observational period n = 7533 very-low-birth-weight infants (VLBWI) with gestational age 22 0/7 to 28 6/7 weeks were enrolled in GNN; n = 1214 VLBWI never received surfactant, n = 2624 VLBWI were treated according to LISA procedure, n = 3695 VLBWI had surfactant via endotracheal tube (ETT). LISA was associated with a reduced risk for adverse outcome measures including mortality [odds ratio (OR) 0.66 (95% CI: 0.51–0.84), p < 0.001] bronchopulmonary dysplasia [BPD; OR 0.55 (95% CI: 0.49–0.62), p < 0.001], intracerebral hemorrhage (ICH) grade II-IV [OR 0.55 (95% CI: 0.48–0.64), p < 0.001] and retinopathy of prematurity [ROP; OR 0.62 (95% CI: 0.45–0.85), p < 0.001]. Notably, LISA was associated with an increased risk for focal intestinal perforation [FIP; OR 1.49 (95% CI: 1.14–1.95), p = 0.002]. The differences in FIP rates were primarily observed in VLBWI born <26 weeks (LISA: 10.0 vs. ETT: 7.4%, p = 0.029). Our observational data confirm that LISA is associated with improved outcome. In infants <26 weeks we noted an increased risk for FIP. Future randomized controlled trials including LISA need to integrate safety analyses for this particular subgroup.
Similar content being viewed by others
Introduction
Less invasive surfactant administration (LISA) to spontaneously breathing preterm infants has been reported to reduce mechanical ventilation, BPD and severe complications of prematurity in randomised controlled trials1,2,3, observational studies4,5,6 and recent meta-analyses7,8,9,10 as compared to intubation for surfactant delivery. Applying LISA to extremely preterm infants followed by non-invasive respiratory support, is a paradigm shift, the proposed effects of which need to be examined carefully. Several factors seem important to achieve a clinical benefit through LISA strategy: infant’s tolerance of surfactant administration while spontaneously breathing, efficacy of continuous positive airway pressure (CPAP)/non-invasive positive pressure ventilation (NIPPV), adequate breathing efforts - supported by methylxanthine treatment to prevent apnea of prematurity - and tolerance of enteral nutrition. Therefore, the LISA strategy represents a bundle of less invasive respiratory care procedures which has been adopted as primary surfactant mode of administration in many German neonatal units. To address further safety aspects of LISA strategy we aimed to evaluate in a large cohort study of the German Neonatal Network (GNN) whether LISA strategy is associated with less frequent complications of preterm birth in most susceptible infants born <29 weeks of gestation.
Results
Primary respiratory care
In our study cohort n = 1214 infants never received surfactant, n = 2624 VLBWI were treated with surfactant according to LISA method, n = 3695 VLBWI had surfactant treatment via endotracheal tube (ETT). As outlined in Table 1, the three groups differed significantly with regard to clinical characteristics. Specifically, infants who received surfactant by LISA or ETT were younger at birth, more often small-for-gestational age and suffered more often from moderate or severe RDS (peak FiO2 in the first 12 h) than infants who received no surfactant. The LISA procedure has been increasingly used in GNN centers during the observational period, e.g. 2009 vs. 2016: 28.7% vs. 50.1% of surfactant treated infants had LISA (Suppl. Figure 1).
LISA is superior to intubation for surfactant delivery for short-term outcomes
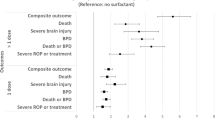
In univariate analyses, LISA was superior to intubation for several clinical outcomes including clinical and culture-confirmed sepsis, pneumonia, higher grade ICH, PVL,ROP, Patent Ductus arteriosus (PDA) surgery, BPD and death but not for FIP and necrotizing enterocolitis (NEC). The proposed benefits of LISA were confirmed in multivariable logistic regression models for clinical sepsis, pneumonia, mortality, BPD, ICH grade II-IV, PVL, PDA and ROP (Table 2).
LISA strategy is associated with focal intestinal perforation in extremely preterm infants
VLBWI without surfactant treatment had a low frequency of FIP (1.2%). VLBWI treated with LISA (4.3%) and VLBWI treated with surfactant via ETT (4.0%) had a comparable FIP rate. In the subgroup of infants with a gestational age <26 weeks, however, LISA treated infants had a higher risk for FIP as compared to infants receiving surfactant via ETT [75/751 (10.0%) vs. 119/1619 (7.4%), p = 0.029; Fig. 1)] but not in the subgroup of infants born 26–28 weeks [39/1873 (2.1%) vs. 30/2081 (1.4%), p = 0.13]. In a multivariable logistic regression analysis, surfactant administration with LISA was associated with an increased risk for FIP [odds ratio (OR) 1.49 (95% CI: 1.14–1.95), p = 0.003] as compared to surfactant treatment via ETT (reference). In a second regression model including further potential confounders such as clinical amniotic infection syndrome, inotropes in the first 24 hours, postnatal steroid exposure or drug PDA treatment we confirmed the independent association of LISA with FIP [OR 1.42 (1.06–1.89), p = 0.018; Fig. 2]. PDA drug treatment (indomethacin or ibuprofen) proved to be a risk factor for FIP [OR 1.53 (1.14–2.06), p = 0.005; Fig. 2]. When PDA treatment with single drugs was included in the regression models, the exposure to indomethacin [OR 1.66 (1.22–2.25), p = 0.001] but not ibuprofen [OR 1.03 (0.77–1.38), p = 0.9] was associated with FIP. However, the effect of LISA on FIP risk remained unchanged, i.e. regression models including indomethacin: OR 1.5 (1.12–2.02), p = 0.006; ibuprofen: OR 1.53 (1.14–2.04), p = 0.004].
Incidence of FIP stratified to primary surfactant management. The figure depicts the percentage of infants suffering from FIP per week of gestation according to exposure to surfactant treatment: Surfactant via ETT (white bars), Surfactant via LISA (grey bars). Numbers of infants are given below each column, i.e. surfactant treated infants via ETT/surfactant treated infants via LISA in each week of gestation.
FIP requiring surgery – logistic regression model. The figure depicts the data of a multivariable logistic regression model to adjust the effect of LISA strategy for known or probable confounding variables including inotropes in the first 24 hours (surrogate marker for severity of primary compromise at birth), amniotic infection syndrome as cause of preterm birth, postnatal steroid hormones (dexamethasone, hydrocortisone, (methyl)prednisolone), PDA drug treatment (indomethacin, ibuprofen), multiple birth, female gender, antenatal steroids, inborn, SGA, gestational age per week. The symbols and lines describe the odds ratios and 95% confidence interval.
Discussion
LISA is a distinct feature of the approach to respiratory care to preterm infants in several countries1,2,3,4,5,6. The LISA procedure has been increasingly used in GNN centers during the observational period. With more than 2500 LISA treated infants this is the largest cohort report so far. We confirmed that LISA is superior to surfactant delivery via ETT with regard to several outcomes related to lung and CNS complications after preterm birth (1–10; 14). In addition, our data suggest beneficial effects regarding the risks of clinical sepsis, pneumonia and higher grade retinopathy. In the subgroup of extremely preterm infants <26 weeks, however, the LISA strategy was associated with a higher incidence of FIP requiring surgery.
FIP is a spontaneous single intestinal perforation typically found at the terminal ileum. Based on previous observational data gestational age is the predominating endogenous risk factor for FIP which occurs in 2–3% of VLBWI and in 5% of extremely-low-birth weight infants (ELBWI; birth weight <1000 g). The median gestational age of affected infants is 25–27 weeks. Male infants are at higher risk than female infants while and infants born SGA have a marked susceptibility to FIP due to predisposition for gut ischemia11,12,13. In addition, administration of postnatal steroids and non-steroidal anti-inflammatory drugs (specifically indomethacin in our setting) has been related to FIP development. Other frequently discussed but not confirmed risk factors for preterm infants are exposure to antenatal steroids, multiple birth and chorioamnionitis12,13,14. Epidemiological data indicate that FIP is associated with significant mortality and long-term morbidity15,16,17,18. In a recent prospective analysis of the Vermont-Oxford Network 19% of infants with laparotomy-confirmed FIP died, with a case-fatality rate of 26–31% in infants with a birth weight <750 g19. Although survival from FIP has increased over the past decades, the pathophysiology of FIP is still not very well understood18. The novel association between LISA and FIP observed in our large cohort needs to be discussed in the context of the complex management of highly susceptible infants. LISA itself may not be causative in the pathogenesis of FIP. A more likely association is that extremely preterm babies (especially those <26 weeks), who would previously have been intubated, are now being managed on CPAP (or NIPPV) at a much earlier and more vulnerable stage than ever before, facilitated by the dose of surfactant given via LISA soon after birth20. Several factors in line with this management need to be considered:
Firstly, maximum CPAP levels (±LISA) applied to highly vulnerable babies may have an impact. In a small retrospective cohort study of VLBWI placed on high noninvasive respiratory support (airway pressure ≥10 cm H2O for at least 12 continuous hours) using nasal continuous positive airway pressure (NCPAP) and/or nasal high-frequency ventilation (NIHFV, n = 70) no increase of FIP was noted20. In line with this, nasal continuous positive airway pressure may affect pre- and postprandial intestinal blood flow velocity in preterm infants21. Hence future prospective studies need to capture the exposure to relatively high positive pressure applied to the upper airway as an input variable.
Secondly, extremely vulnerable infants are exposed to several modulators of mucosal integrity including devices (ventilation support, gastric tubes), bacterial colonization, nutrition and drugs. The timing of treatment strategies - not only drugs (non-steroidal anti-inflammatory drugs, steroids) but also implementation of invasive measures (e.g. tracheal ventilation) - might be critical for FIP risk.
Thirdly, abdominal distension of the gastrointestinal tract due to delayed meconium passage and CPAP may result in increased shear forces and stretching of the intestinal mucosa. The texture of the gastric tube (size of side holes, closed or open tip) may be important for adequate aspiration of gas and stomach content in order to avoid massive dilatation of bowels. Finally, center specific aspects may also be significant, i.e. centers use different strategies in treating RDS and apnea/bradycardia syndrome with regard to the time point of secondary tracheal ventilation, particularly in infants <26 weeks. In line with this, the frequency of episodes with relevant desaturations might contribute to FIP risk via temporary hypoxia of the gut. Our data provide a basis for benchmarking and critically reviewing all aspects of less invasive surfactant application strategies. Strengths of our study are the large sample size and prospectively recorded datasets. With increasing expertise of NICUs in the technique and evidence that LISA is highly beneficial for several outcomes, we noted that an increasing number of infants <26 weeks are managed as such22. The major limitations of our study are the post-hoc analysis and the observational design. We are not able to rule out the possibility of unrecognized confounders [e.g. approach to neonatal resuscitation, changing strategies of PDA-management (ibuprofen, indomethacin, paracetamol), center aspects] which might bias the results of our analysis. Whether “protective” or earlier intubation of extremely preterm infants with significant abdominal distension is beneficial, needs to be subject of further trials. In line with this, animal models such as preterm lambs may complement clinical investigations and guide future research to evaluate underlying causes of our observation23.
In conclusion, our observational data confirm that LISA is associated with improved outcome. In highly preterm infants <26 weeks we noted an increased risk for FIP. Future randomized controlled trials including LISA strategy need to integrate safety analyses for this particular subgroup, and clinicians need to balance the optimal time point of secondary tracheal ventilation in extremely preterm babies initially managed with LISA.
Methods
The GNN is a population-based cohort study of VLBWI enrolled in 54 neonatal intensive care units in Germany (GNN). The data for this observational investigation were collected between the 1st of January 2009 until the 31st of December 2016. After written informed consent was given by the parents, infants were enrolled in the GNN by the attending physicians. Then a predefined clinical data set was recorded on case report forms and sent to the GNN coordinating center in Lübeck. The inclusion criteria for this observational study were infants with a birth weight <1500 g and gestational age ≥22 0/7 and <29 weeks who received primary intensive care. We excluded VLBWI who were previously enrolled in RCTs evaluating LISA strategy, i.e. AMV and NINSAPP1,2, and infants with lethal malformations.
A physician or study nurse from the central GNN office (University of Lübeck) with expertise in neonatology monitored the data quality by annual site visits. Clinical data were coded and entered into a central database. Written parental consent was obtained by parents or caregivers. The GNN study was approved by the ethics committee at each participating centre.
Ethics
All experimental protocols were approved by the ethics committee of the University of Lübeck(08–022) and the local ethical committees at each study center. Informed consent was obtained from all subjects. All methods were carried out in accordance with relevant guidelines and regulations, specifically: the Declaration of Helsinki, the current revision of ICH Topic E6, the Guidelines for Good Clinical Practice, and the Guidelines of the Council for International Organization of Medical Sciences, the WHO (“Proposed International Guidelines for biomedical research involving human subjects”).
Definitions
Outcomes
Clinical sepsis was defined as sepsis with at least two signs (temperature >38 °C or <36.5 °C, tachycardia >200/min, new onset or increased frequency of bradycardias or apneas, hyperglycemia >140 mg/dl, base excess <−10 mval/l, changed skin color, increased oxygen requirements) and one laboratory sign (C-reactive protein >1 mg/dl, immature/neutrophil ratio >0.2, white blood cell count <5/nl, platelet count <100/nl) and antibiotic treatment for ≥5days, but no proof of causative agent in the blood culture. Blood-culture confirmed sepsis was defined as clinical sepsis with proof of causative agent in the blood culture24.
Pneumonia was defined as infection with at least one radiological sign (e.g. infiltrate on X-ray) or new deterioration of gas exchange (frequent desaturations, increased oxygen requirements) and at least four clinical/laboratory signs, i.e. tachycardia >200/min, new onset or increased frequency of bradycardias or apneas, new onset of tachypnea/dyspnea, putrid tracheal aspirate, more frequent need of tracheal aspiration, temperature instability; C-reactive protein >2 mg/dl, immature/neutrophil ratio >0.224.
Moderate to severe intracerebral haemorrhage (ICH) was defined as grade II–IV ICH according to Papile25.
Periventricular leukomalacia (PVL) was defined as white-matter brain injury, characterized by cystic degeneration of white matter near the lateral ventricles as diagnosed by ultrasound imaging which was applied in all participating centres.
Retinopathy of prematurity (ROP) was defined as higher stage ROP requiring intervention (crytherapy, laser therapy or anti-VEGF treatment).
FIP requiring surgery was defined as the occurrence of spontaneous intestinal perforation with the need for laparotomy and the macroscopic diagnosis of isolated FIPs as described by the attending surgeon.
NEC requiring surgery was defined as clinical NEC classified as Bell Stage II or Bell Stage III with the need for laparotomy with or without resection of necrotic gut, and the macroscopic diagnosis of NEC.
Patent ductus arteriosus (PDA) surgery was defined as required surgical ligation of PDA.
Bronchopulmonary dysplasia (BPD) was defined as need for oxygen supplementation or ventilation support at 36 weeks corrected age.
Death was defined as mortality during primary stay in hospital.
Clinical Parameters
Gestational age was calculated from the best obstetric estimate based on early prenatal ultrasound and obstetric examination. Small for gestational age was defined as birth weight percentile <10 according to gestational age26.
Severity of RDS was characterized as categories of peak FiO2 in the first 12 h, i.e. mild RDS: FiO2 0.21–0.39, moderate RDS: FiO2 0.4–0.59, severe RDS: FiO2 ≥0.6.
Drug treatment of Patent Ductus arteriosus (PDA) was defined as treatment of PDA with ibuprofen or indomethacin.
Postnatal steroid treatment was defined as any systemic treatment with steroids (hydrocortisone, (methyl-) prednisolone or dexamethasone).
Statistical analysis
Univariate analysis: Study populations were compared using univariate techniques. Continuous variables (gestational age, birth weight, Apgar scores) were evaluated with Mann-Whitney-U test. Categorical variables (e.g. gender) were evaluated with a two-tailed Pearson-Chi-square test.
Multivariate analysis: Logistic regression analyses were performed for all outcomes subjected to univariate analysis to adjust the effect of LISA for known confounding variables, particularly gestational age per week, small-for-gestational age, inborn, antenatal steroids, gender and multiple birth. Mode of surfactant administration was included as an independent categorical variable with surfactant treatment via ETT as reference. To address independent factors associated with FIP we additionally included reported or assumed risk factors for FIP requiring surgery based on literature11,12,13,27 such as amniotic infection syndrome as cause of preterm birth, inotropes in the first 24 hours, exposure to postnatal steroids and exposure to drug treatment of PDA. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A p-value of <0.05 was considered statistically significant. Missing data were not imputed. Data analysis was performed using the SPSS 22.0 data analysis package (Munich, Germany).
References
Göpel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131, e502–9 (2013).
Kribs, A. et al. Non-intubated surfactant application during CPAP-assisted spontaneous breathing versus conventional therapy in extremely preterm infants – a randomised controlled trial. JAMA Pediatrics 169, 723–30 (2015).
Dargaville, P. A., Aiyappan, A., Cornelius, A., Williams, C. & De Paoli, A. G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 96, F243–8 (2011).
Dargaville, P. A. et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 98, F122–6 (2013).
Klebermass-Schrehof, K. et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology 103, 252–8 (2013).
Isayama, T., Iwami, H., McDonald, S. & Beyene, J. Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 316, 611–24 (2016).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 102, F17–F23 (2017).
Lau, C. S. M., Chamberlain, R. S. & Sun, S. Less Invasive Surfactant Administration Reduces the Need for Mechanical Ventilation in Preterm Infants: A Meta-Analysis. Glob Pediatr Health 4, 2333794X17696683 (2017).
Rigo, V., Lefebvre, C. & Broux, I. Surfactant instillation in spontaneously breathing preterm infants: a systematic review and meta-analysis. Eur J Pediatr 175, 1933–1942 (2016).
Koshinaga, T. et al. Spontaneous localized intestinal perforation and intestinal dilatation in very-low-birth-weight infants. Acta Pediatrica 95, 1381–88 (2006).
Wadhawan, R. et al. Spontaneous intestinal perforation in extremely low birth weight infants: association with indometacin therapy and effects on neurodevelopmental outcomes at 18-22 months corrected age. Arch Dis Child Fetal Neonatal Ed 98, F127 (2013).
Shorter, N. A. et al. Indomethacin-associated bowel perforations. A study of possible risk factors. J Pediatr Surg 34, 442–44 (1999).
Gordon, P. V. & Attridge, J. T. Understanding clinical literature relevant to spontaneous intestinal perforation. Am J Perinatol 26, 309–316 (2009).
Roze, E. et al. Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr Res 70, 619 (2011).
Shah, T. A. et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol 32, 552 (2012).
Härtel, C. et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J Pediatr 165, 285–289 (2014).
Gordon, P. V. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res 65, 138–144 (2009).
Fisher, J. G. et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg 49, 1215e9 (2014).
Binmanee, A., El Helou, S., Shivananda, S., Fusch, C. & Mukerji, A. Use of high noninvasive respiratory support pressures in preterm neonates: a single-center experience. J Matern Fetal Neonatal Med 7, 1–6 (2017).
Havranek, T., Madramootoo, C. & Carver, J. D. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J Perinatol 27, 704–8 (2007).
Mehler, K. et al. Survival among infants born at 22 or 23 weeks’ gestation following active prenatal and postnatal care. JAMA Pediatr 170, 671–7 (2016).
Niemarkt, H. J. et al. Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res 76, 166–70 (2014).
Leistner, R., Piening, B., Gastmeier, P., Geffers, C. & Schwab, F. Nosocomial infections in very low birthweight infants in Germany: current data from the National Surveillance System NEO-KISS. Klin Padiatr 225, 75–80 (2013).
Papile, L., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage. A study of infants with birth weights less than 1,500 gm. J Pediatr 92, 529–534 (1978).
Voigt, M. et al. Analyse des Neugeborenenkollektivs der Jahre 1995-1997 der Bundesrepublik Deutschland. 11. Mitteilung: Unterschiede im somatischen Entwicklungsstand Neugeborener unter Berücksichtigung des Herkunftslandes der Mutter. Geburtsh. Frauenheilk. 66, 391–9 (2006).
Göpel, W. et al. German Neonatal Network (GNN). Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr 104, 241–6 (2015).
Acknowledgements
The writing group for this article acknowledges the contributions of all other members of the German Neonatal Network. We are grateful to the infants, parents and health care providers who supported our study. The German Neonatal Network is funded by the German Ministry for Education and Research (BMBF-grant-No: 01ER0805 and 01ER1501).
Author information
Authors and Affiliations
Contributions
Christoph Härtel wrote the first draft of the manuscript, no honorarium, grant, or other form of payment was given to anyone to produce the manuscript. Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. C.H. and W.G. conceptualized and designed the study, carried out the initial data analyses, supervised and coordinated the data collection, drafted the initial manuscript, and approved the final manuscript as submitted. P.P., K.H., A.H., A.K., K.M., C.R., C.W., M.V., E.H. supported the study design and the development of data collection instruments coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing Interests
Christoph Härtel, Egbert Herting and Wolfgang Göpel have received funding for a clinical study and have received payments for advisory board duties from Chiesi Pharmaceuticals, Parma, Italy. All other authors have indicated that they have no financial relationships relevant to this article to disclose.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Härtel, C., Paul, P., Hanke, K. et al. Less invasive surfactant administration and complications of preterm birth. Sci Rep 8, 8333 (2018). https://doi.org/10.1038/s41598-018-26437-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26437-x
This article is cited by
-
Wide use of broad-spectrum antibiotics in very low birth weight infants with spontaneous focal intestinal perforation—is it really justified?
Infection (2024)
-
Should less invasive surfactant administration (LISA) become routine practice in US neonatal units?
Pediatric Research (2023)
-
Clinical impact of less invasive surfactant administration using video laryngoscopy in extremely preterm infants
Pediatric Research (2023)
-
Evaluation of a respiratory care protocol including less invasive surfactant administration in preterm infants
Pediatric Research (2023)
-
Wandel der neonatologischen Versorgung
Die Ophthalmologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.