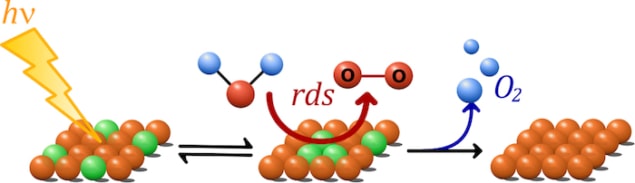
A new study of water oxidation on the surface of iron oxide (Fe2O3) could lead to the development of more efficient water splitting devices. The work, which is a combined experimental and theory study, reveals that water splitting on Fe2O3 changes with light intensity and that the rate of water oxidation increases with the third power of the surface hole density.
An enzyme called photosystem II (PSII) in plants oxidizes water in the presence of sunlight to split water. This natural catalytic process extracts electrons and protons from water molecules and produces molecular oxygen – a process that has allowed for life to flourish on Earth. The electrons are immediately used to make a chemical fuel called adenosine triphosphate (ATP) that the plant stores and utilizes when needed.
Researchers are trying to mimic these natural photosynthetic mechanisms to harness sunlight and split water into molecular oxygen and hydrogen, or to reduce carbon dioxide to carbon-based molecular fuels. α-Fe2O3 (or haematite) is a promising material for making photoelectrodes for this application, but its catalytic functionality is little understood at the molecular level.
Measuring the kinetics of water oxidation
A team led by James Durrant at Imperial College London, Victor Batista at Yale University, Michael Grätzel of the Swiss Federal Institute of Technology Lausanne and Erwin Reisner at the University of Cambridge has now used optical spectroscopy to measure how the kinetics of water oxidation changes as more holes accumulate on the haematite surface when it exposed to sunlight. The researchers had already employed this technique in previous work to determine rate laws for water and methanol oxidation and proton reduction on various photoelectrocatalytic surfaces.
The technique allowed them to determine rate laws and rate constants for the water oxidation reaction and determine, for example, how many holes have to aggregate to access the rate-limiting step of the reaction and the activation energy for this reaction.

Ruthenium catalyst sets new efficiency record for water splitting
The new experimental studies, undertaken by Camilo Mesa and Laia Francàs at Imperial, show that water oxidation on iron oxide changes with light intensity and that the rate of oxidation increases with the third power of the density of holes on the surface of Fe2O3. “This implicates the equilibria of three holes with the water oxidation catalytic centre, reducing the activation energy for the water oxidation and appears to be a key factor in other studied metal oxides,” explains Mesa.
Thanks to accompanying theoretical work by Yales Ke Yang, the team identified the catalytic sites under low light intensity of an oxidizing Fe(OH)-O-Fe(OH) catalytic core that can extract electrons from water by accumulating up to three oxidizing equivalents (missing electrons or holes) under solar irradiation of 1 sun. This mechanism is similar to the proposed activation catalytic site in PSII, they say – even though haematite and PSII are radically different in composition and structure.
Full details of the research are reported in Nature Chemistry.