Abstract and Introduction
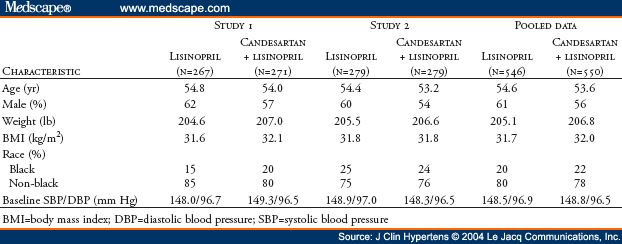
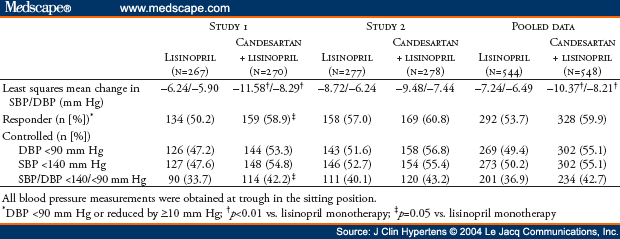
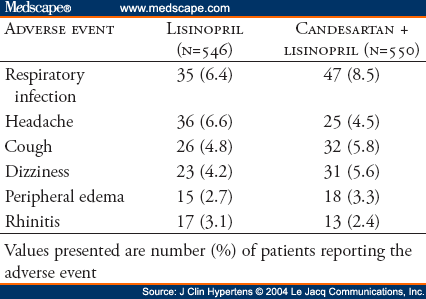
The AMAZE (A Multicenter Trial Using Atacand and Zestril vs. Zestril to Evaluate the Effects on Lowering Blood Pressure) program included two identical studies sponsored by AstraZeneca LP. The oral form of candesartan is candesartan cilexetil; for simplicity, the term "candesartan" is used throughout this manuscript. Two identical multicenter, randomized, double-blind studies were performed to determine if addition of the angiotensin receptor blocker candesartan was more effective in lowering blood pressure than up-titration of lisinopril. Hypertensive patients (N=1096) who were uncontrolled on lisinopril 20 mg daily were randomized (1:1) to receive either 8 weeks of high-dose lisinopril (40 mg) or the addition of candesartan (16 mg) for 2 weeks followed by 32 mg for 6 weeks. Study 1 (n=538) demonstrated decreases in trough sitting systolic/diastolic blood pressures at Week 8 by 6.2/5.9 mm Hg, respectively, for the lisinopril up-titration treatment group and by 11.6/8.3 mm Hg, respectively, for the lisinopril plus candesartan treatment group (p<0.01 in comparing both blood pressures reductions between the two treatment groups). Corresponding results for Study 2 (n=558) are reductions of 8.7/6.2 mm Hg and 9.5/7.4 mm Hg, respectively, for each of the two treatment groups. For Study 2, comparisons of systolic/diastolic blood pressures between the two treatment groups were not statistically significantly different (p = 0.51/p = 0.08, respectively). Post hoc pooled analysis (N=1096) demonstrated a slightly greater blood pressure reduction with lisinopril plus candesartan compared with lisinopril (3.1/1.7 mm Hg). A 95% confidence interval limit for the difference in least squares mean change from baseline in systolic blood pressure between the two treatment groups is -4.8 to -1.5 and is -2.8 to -0.7 in mm Hg for diastolic blood pressure. The blood pressure control rates (<140/<90 mm Hg) were 42.7% and 36.9%, respectively. Both treatment regimens were well tolerated in all groups. In conclusion, for hypertensive patients not controlled by lisinopril 20 mg once daily, addition of candesartan (32 mg once daily) or doubling the dose of lisinopril provides safe, additional reduction of blood pressure.
Combination therapy is now recommended for most people with hypertension, in part because the control rates for hypertension (>140 mm Hg systolic and >90 mm Hg diastolic) are still only 34% in hypertensive adults aged 18 to 74 years.[1] Among the most popular antihypertensive drugs are agents that block the renin-angiotensin-aldosterone system (RAAS), including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs).[2,3,4,5] ACE inhibitors block the main pathway for conversion of angiotensin I to the potent vasoconstrictor and pressor agent, angiotensin II, but the discovery of species-specific alternate pathways for angiotensin II generation raises the possibility that ACE inhibitors do not fully block the RAAS under all circumstances.[6,7] ACE inhibitors also block the degradation and inactivation of kinins and other biologically active peptides,[3] and cause cough and other side effects.[8,9] In contrast, ARBs selectively block the AT1 receptor subtype, thereby inhibiting the effects of angiotensin II regardless of the pathway leading to its generation. Adverse event rates for ARBs are low, and their tolerability profiles are similar to that of placebo.[10] Some investigators have postulated that the addition of an ARB to an ACE inhibitor may allow more complete blockade of the RAAS pathway than either drug alone,[7] potentially leading to improved blood pressure (BP) control.[11,12,13,14,15] Alternatively, it may be possible to improve the effectiveness of ACE inhibition by simple dose titration.
This report summarizes the results of the AMAZE (A Multicenter Trial Using Atacand and Zestril vs. Zestril to Evaluate the Effects on Lowering Blood Pressure) program, which included two identically designed multicenter, randomized, double-blind studies conducted with two separate cohorts of investigators, according to guidelines and recommendations from the US Food and Drug Administration. To reduce the risk of a false-positive study, we elected to conduct two independent studies, each at a 0.05 alpha level. AMAZE sought to determine whether addition of the ARB candesartan (16 mg titrated to 32 mg daily) to lisinopril therapy (20 mg daily) is more effective in lowering BP than titration of lisinopril to 40 mg daily in hypertensive patients uncontrolled by lisinopril 20 mg daily.
© 2004 Le Jacq Communications, Inc.
Cite this: Antihypertensive Efficacy of Candesartan-Lisinopril in Combination vs. Up-Titration of Lisinopril: The AMAZE Trials - Medscape - Sep 01, 2004.