Moderna May Supply 40M Coronavirus Vaccine Doses to Japan
Moderna, Inc. MRNA announced that it is currently engaged in discussion with the Ministry of Health, Labour and Welfare of Japan ("MHLW") for a potential supply of 40 million doses of its coronavirus vaccine candidate, mRNA-1273, if approved. Japan is looking to provide vaccines against COVID-19 to the public as soon as possible.
Per the terms of the agreement, Moderna will be responsible for the supply of the vaccine, while Japan-based pharmaceutical company, Takeda Pharmaceutical TAK will distribute the vaccines in the country. The supply of the vaccine is anticipated to begin in the first half of 2021, only if the candidate receives approval.The candidate is currently being evaluated in a phase III study.
Moderna and other leading coronavirus vaccine-makers are currently on a signing spree for supply of their respective coronavirus vaccines to different countries, following their potential approval. Earlier this month, Moderna signed an agreement with the United States for supply of 100 million doses of mRNA-1273. The deal also includes an option for the federal government to purchase up to an additional 400 million doses. The company confirmed last week that it is in advanced discussion with the European Commission to supply 80 million doses of coronavirus vaccine candidate. If the deal with the European Commission is successful, it will also include an option to purchase additional 80 million doses of mRNA-1273 by the member states of European Union. Moderna is scaling up global manufacturing capacity to support its delivery target of approximately 500 million doses per year and possibly up to 1 billion doses per year, beginning in 2021.
Meanwhile, the United States has advanced purchase agreements with Johnson & Johnson JNJ, AstraZeneca, Pfizer and BioNTech SE BNTX, and Sanofi and GlaxoSmithKline for their respective vaccine candidates. Some of these companies have also signed supply deals with Europe and Japan. We note that the majority of governments are trying to secure vaccine doses for their people as soon as they are approved. Hence, we expect more supply deals to be signed across the globe.
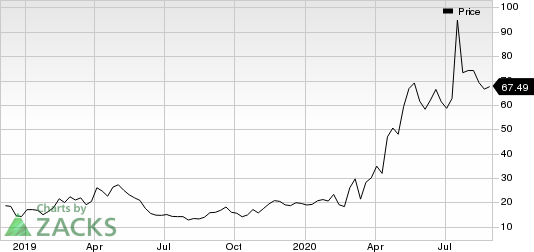
Shares of Moderna have skyrocketed 245% so far this year compared with the industry’s increase of 1.2%.

Moderna has received at least $2.48 billion of funding from the U.S. government in several tranches to support the development as well as potential commercialization of its coronavirus candidate. The company is evaluating 100 micrograms dose of mRNA-1273 administered on a two-dose vaccination schedule, given 28 days apart for safety and reactogenicity in the phase III study. Enrollment is expected to be completed in September. Additionally, in July, Moderna reported positive updated interim data from a phase I study on mRNA-1273. The results demonstrated that participants vaccinated twice with mRNA-1273 led to rapid and strong immune responses against SARS-CoV-2 through day 57 across all three dose cohorts.
Zacks Rank
Moderna currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth.
Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Click to get this free report Johnson Johnson (JNJ) : Free Stock Analysis Report Moderna, Inc. (MRNA) : Free Stock Analysis Report BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report Takeda Pharmaceutical Co. (TAK) : Free Stock Analysis Report To read this article on Zacks.com click here. Zacks Investment Research
